
The recurring controversy and politicization on the use of anabolic androgenic steroids (AAS) has been front and center in the news headlines. Within the last month the release of the book, “Wada MF, Williams, L. Game of Shadows: Barry Bonds, BALCO, and the Steroids Scandal that Rocked Professional Sports. Gotham Books; March 23, 2006,” precipitating Commissioner Bud Selig to name George J. Mitchell, former Senate majority leader, to lead an investigation into what appears to be the sport’s long, and troubling, involvement with steroids. This falls almost exactly one year after publication of the book, “Canseco, J. Juiced: Wild Times, Rampant ‘Roids, Smash Hits, and How Baseball Got Big. Regan Books; February 14, 2005.” Following within a month was the Government Reform Committee Hearing, United States House of Representatives, on March 17, 2005. The hearing was entitled “Restoring Faith in America’s Pastime: Evaluating Major League Baseball’s Efforts to Eradicate Steroid Use.”[[1]] The hearing was the first in a series of hearings regarding steroid use in professional sports.
Since the introduction of steroids into mainstream culture, the media, sports organizations, medical community and public have all expressed their values and judgment of their ethical use outside of medical necessity. Within the medical establishment there is a pervasive atmosphere of fear and intimidation towards physicians who treat AAS users or prescribe AAS. This has created a vacuum or void in the proper use of AAS; an abandonment of basic scientific principles; and an ever increasing population of men at risk for significant health problems. For the greater part of 10 years I have found that the medical treatment provided for the condition termed anabolic steroid induced hypogonadism (ASIH), is nonexistent or ignored by the great majority of medical professionals. As predicted since my entry into this field in 1995 more and more cases of ASIH would appear due to this negligence. Clear and convincing evidence of this is demonstrated by recent articles in peer-reviewed medical literature affirming concerns for the long term effects of untreated ASIH [[2]], rapidity and severity of symptoms in ASIH [[3]], and inappropriate treatment with AAS based upon a flawed clinical study design [[4]].
An unproven and unfounded assumption has been made in the medical establishment that the treatment for an individual suffering from ASIH is to do nothing which is coined ‘watchful waiting’ and in time HPTA functioning will return to normal. This premise can be traced back to Knuth et al. (1989) [[5]] studying semen parameters in AAS users. He concluded, “Results suggest that even after prolonged use of extremely high doses of anabolic steroids, sperm production may return to normal.” The ability to create spermatozoa does not equate with a normal functioning HPTA. Hypogonadal males are known to have the ability to produce spermatozoa. There are no studies that demonstrate that serum testosterone levels sufficient for spermatozoa production are positively associated with the clinical effects of testosterone elsewhere within the individual. At the very same time members of the medical community announce an alert to suicide risk after AAS cessation. Kirk J. Brower, M.D. from the University of Michigan stated, “… whereas depressive episodes and suicide attempts are most likely to occur within three months of stopping AAS use.”[[6]] Shortly thereafter Texas HB 3563, “Use Of Anabolic Steroids By Public School Students,” was passed and signed into law June 18, 2005. Of particular importance is the bill analysis citing the problem of “clinical depression when steroid use is stopped.” [[7]] The obvious question is who are these astute physicians that are able to know the individual to attempt suicide during the treatment plan of ‘watchful waiting’ or do nothing?
AAS have proven beneficial in treating numerous medical conditions and symptoms in ailing populations. Currently HIV males account for an estimated 560,000 people in the U.S. reports the Centers for Disease Control and Prevention. Those experiencing lean muscle wasting greater than 10% will likely be administered a form of AAS therapy to help retain fat free tissue. According to the U.S. Food and Drug Administration, approximately 5 million men in the U.S. are considered hypogonadal with roughly 250,000 being treated with testosterone replacement. Finally, as of 2002 over 14 million men suffer with osteoporosis and low bone mass according to the National Osteoporosis Foundation. Cumulatively 810,000 people are possibly being treated with some type of androgen or AAS. Add to these patients the countless numbers of adolescents, young and middle aged men, and athletes using AAS for cosmetic and athletic enhancement the potential population of at risk men numbers well over one million.
ANDROGENIC ANABOLIC STEROIDS (AAS)
Testosterone and testosterone analogues, anabolic-androgenic steroids (AAS), have long been used in the athletic community for improving lean muscle tissue and strength. A positive correlation has been shown with testosterone to include: increased protein synthesis resulting in lean muscle tissue development [[8]], enhanced sexual desire (libido) [[9]], increased muscular strength [[10]], increased erythropoiesis [[11]], a possible positive effect on bone development [[12]], improved mental cognition and verbal fluency [[13]], and male masculinizing characteristics [[14]]. Recently, however, clinicians have recognized the potential benefits of their use in the treatment of various conditions and ailments. Numerous studies have discussed the use of AAS in the treatment of HIV-associated conditions [[15]], hypogonadism [[16]], impotence [[17]], burn victims [[18]], various anemia’s [[19]], deteriorated myocardium [[20]], glucose uptake [[21]], continuous ambulatory peritoneal dialysis (CAPD) [[22]], alcoholic hepatitis [[23]], hemochromatosis [[24]] and prevention of osteoporosis [[25]]. Since there has been such strong evidence for the medicinal use of AAS in the treatment of various conditions, these medications have become more prevalent in the medical community.
While the use of AAS by physicians has become more prevalent, this class of medicines is not without their inherent problems. AAS have been shown to induce hypogonadotropic hypogonadism [[26]]. This condition typically results from an abnormality in the normal functioning of the hypothalamic-pituitary-gonadal axis (HPTA), either from an over-or underproduction of one of the hormone secreting glands, causing a cascading unbalance in the rest of the axis. This condition may be the result of a physiological abnormality (i.e. mumps orchitis, Klinefelters syndrome, pituitary tumor) or as an acquired result of exogenous factors (i.e. androgen therapy, anabolic-androgenic steroid administration). Clerico et al found a dramatic suppression of serum gonadotropin levels in athletes given methandrostenolone, suggesting a direct action of AAS on the hypothalamus [[27]]. Similar results of suppressed gonadotropins have been found in patients supplementing solely testosterone [[28]]. Case report studies discussed a 36-year old male competitive bodybuilder and a 39-year old father, each using various AAS regimens over extended periods of time, who showed a blunted response to GnRH stimulation tests [[29]]. Bhasin et al showed a complete suppression of serum luteinizing hormone levels after administration of 600 mg testosterone enanthate over ten weeks [[30]]. A similar study administered 600 mg of nandrolone decanoate to 30 HIV-positive males over twelve weeks [[31]]. The results documented mild elevations in hemoglobin and alanine aminotransferase levels but no reference to LH or testosterone levels. The lack of gonadotropin response is puzzling as the data showed 12 of 30 subjects experienced testicular shrinkage, implying Leydig cell dysfunction and suppressed testosterone levels. A contraceptive investigation found that 6 of 9 men receiving 200mg of testosterone enanthate per week became azoospermic with suppressed gonadotropin levels after 16-20 weeks [[32]]. Other studies using AAS also showed no reference to LH or FSH levels but suppressed values are expected in each case [[33]].
Declining, or suppressed, circulating testosterone levels as a result of either pathophysiological or induced hypogonadal conditions can have many negative consequences in males. Declining levels of testosterone have been directly linked to a progressive decrease in muscle mass [[34]], loss of libido [[35]], decrease in muscular strength [[36]] impotence [[37]], oligospermia or azoospermia [[38]], increase in adiposity [[39]] and an increased risk of osteoporosis [[40]].
CHRONOLOGY
In 1982, more than two decades ago, it was shown that nandrolone decanoate caused a suppression of the HPTA in males. [[41]] A 1989 study demonstrated the period of hypogonadism after androgenic-anabolic steroid cessation in male hemodialysis patients.[[42]] The authors warned that the cessation of anabolic steroids caused hypogonadism stating: “Nandrolone decanoate are anabolic steroids prescribed for uremic anemia and those may possibly exacerbate uremic gonadal damage. This clinical study suggests that some anabolic steroids play a role in uremic hypogonadism.”
The sequence of changes in body composition induced by testosterone and reversal of changes after cessation was studied in 1992. Testosterone treatment produced a progressive increase in lean body mass and a progressive decrease in body fat. After the testosterone was stopped a period of hypogonadism ensued and the body composition reverted slowly back to normal. [[43]]
Each of the studies done prior to 1995 is designed correctly taking into consideration the characteristics of life. The characteristics of life are to physiology as Newton’s Laws of Motion and Gravity are to physics. If one was to disregard or fail to consider the Law of Gravity in a physics experiment the conclusions drawn from such a study would be erroneous and wrong. The Characteristics of life are the following: All living things follow the tenets of cell theory; Living things acquire and use energy and produce wastes; Living things reproduce, grow, and develop; Living things evolve;. Living things respond to stimuli; Living things maintain a state of homeostasis; All living things are made up of some kind of atoms and molecules. The scientific method is a method of collecting evidence through observation, questioning, hypothesis formation, and hypothesis testing. Similarly, if one was to disregard or fail to consider the characteristics of life in a physiology experiment their conclusions would be erroneous and wrong.
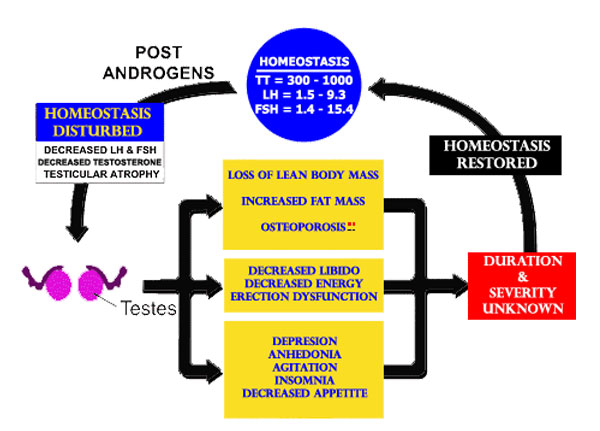
THE FAILURE TO ACCOUNT FOR HOMEOSTASIS WOULD BE THE EQUIVALENT OF STATING GRAVITY DOES NOT EXIST.
MIND WARP
Ironically it was not until 1996 and the publication by Bhasin [[44]] that the medical community finally came to recognize that androgens do enhance the ability of the body to manufacture muscle. Bhasin failed to document and report the effects for the period following cessation of testosterone. Since its publication, the same experimental protocol with no follow up has been used in many patient populations by many different researchers. Uniformly publications have reported positive body composition changes with AAS administration but neglect to include any follow-up on duration and severity of ASIH after AAS cessation. [[45]]
That it took over 60 years since the discovery of the hormone testosterone and countless years of unsupported comments by the pundits of the exact opposite nature of testosterone is a clear indication of medicine’s lack of intellectual and clinical curiosity in the face of a highly politicized and rhetoric laden class of drugs. This lack of rigor and adherence to scientific principles persisted and researchers made conclusions which were erroneous, flawed, and simply wrong. This could not have been better displayed than by researchers studying AAS in hemodialysis patients.
Navarro JF et al. (1998) concluded androgen administration had beneficial effects on erythropoiesis, as well as positive anabolic actions in patients under peritoneal dialysis. [[46]] Gascon A et al. (1999) concluded, “The use of Nandrolone decanoate will allow us an acceptable treatment of anemia, as well as a better nutritional condition in elderly patients on dialysis.” [[47]] Lastly, Johansen KL et al. (1999) concluded, “Treatment with Nandrolone for six months resulted in a significant increase in lean body mass associated with functional improvement in patients undergoing dialysis.” If you look at testosterone and luteinizing hormone values at baseline, each decreased significantly at three months. [[48]] None of these published studies noted or referenced the previous work cited above that studied AAS in hemodialysis patients.
ADVERSE EVENTS
In the paper by Pena et al. many of the adverse events associated with ASIH are displayed. But even more remarkable is that the ignorance and unfamiliarity with AAS is there for all to see in a Board certified endocrinologist and urologist.
The patient was an HIV+ married male discovered to be azoospermic when the couple was exploring artificial insemination as an option to have children. His medications included testosterone enanthate and oxandrolone. To restore spermatogenesis the urologist discontinued only the testosterone and allowed the patient to remain on oxandrolone. Within months of this action the patient’s testosterone level was 30 nanograms per deciliter, with luteinizing hormone (LH) and follicle-stimulating hormone (FSH) both below normal range, and suffering from notable depression and irritability that necessitated antidepressant medication. A repeat semen analysis continued to demonstrate azoospermia.
At this point the patient was referred to a medical endocrinologist for the evaluation of central hypogonadism. Pituitary and thyroid disorders were ruled out by normal serum prolactin and thyroid hormone levels, respectively. Magnetic resonance imaging of the brain and pituitary were normal. Finally, a decision was made that the patient’s continued hypogonadism after testosterone cessation was due to the oxandrolone. After discontinuing both testosterone enanthate and oxandrolone for three months the patient’s serum testosterone rose to 134ng/dL which was sufficient for the production of spermatozoa. The patient was then encouraged to restart his androgen supplementation to improve both physical and emotional well-being.[[49]]
The evaluation and management of this patient was extraordinarily poor and inept. First, it is incredulous that these physicians are apparently unfamiliar with oxandrolone. Despite this they continued to treat him and order test which are costly and unnecessary. The MRI was without any medical indication, particularly in the face of the known medications testosterone and oxandrolone. It is fortuitous that the MRI was negative since ~10% of the general populations have asymptomatic pituitary adenomas.
This patient demonstrates the pervasive effect upon the health and welfare those AAS studies which failed to account for homeostasis. Clinicians across the USA and beyond are using these studies as a basis for the clinical care of patients. That neither of these physicians even knew the most rudimentary AAS knowledge and was unaware as to ASIH after AAS cessation is horrific and shocking. But what is particularly disheartening is no one displayed any sense on what to do regarding the patient’s HPTA. There are literally tens of thousands of patients in the United States who are receiving similar androgen treatment as the patient in Pena et al., each is potentially being left in the state of HPTA dysfunction.
LONG-TERM EFFECTS
Urhausen et al. (2003) studied serum parameters in 15 AAS users. The mean time after steroid cessation was 43 months with the minimum length of time 1 year and the maximum 10 years in the study. The average amount of medication used was a mean of 700 milligrams for 26 weeks, half a year, for 9 years. [[50]] The long-term side-effects of anabolic steroid use were demonstrated to be most pronounced on the HPTA. It was found A13/15 ex-AAS users were found in the lower 20 percent of the normal reference range for testosterone, 2/15 ex-AAS users were found below the normal range with values of 6.6 and 9.0 nanomoles per liter.
vanBreda et al. (2003) presents a case study in a 37y male who after AAS cessation had persistent HPTA dysfunction. [[51]] Restoration of HPTA dysfunction was achieved with the use of LH-RH.
DURABILITY
In 2004, Schroeder et al. included an equivalent amount of time for follow-up after AAS cessation as AAS administration. The study found that the positive body composition changes produced by the androgen in the study had completely disappeared after cessation. This was due to the state of hypogonadism induced by the administration of androgens (ASIH). Anabolic improvements were lost 12 weeks after discontinuing the androgen.[[52]]
The publication and timing of the study by Schroeder et al. is strongly suspect. This study may have never possibly been done if not for a formal complaint filed against the researchers through the Office of Human Research Protection (OHRP).[[53]] Also, documents received from researchers through the Freedom of Information Act (FOIA) conflict with data observed in the published study. Over 200+ pages were clearly missing in the materials sent.
HPTA NORMALIZATION & RESTORATION
 TREATMENT
TREATMENT
Autonomy
There are vast differences between the health of an individual with frank hypogonadism (primary hypogonadism or testicular failure; secondary hypogonadism – hemochromatosis, Kleinfelters, etc.) and the individual with Andropause or PADAM (Partial Androgen Deficiency in Aging Male). The morbidity observed with true hypogonadism have been documented. While there are clinical indicators that are improved with AAS administration in Andropause there are no studies to show that these are factors for increased morbidity or an overall decreased quality of life. Until these studies are done care should be taken regarding the continuous long term administration of AAS.
There are also clinical situations which would necessitate AAS cessation for health concerns. With increasing AAS use these clinical conditions are sure to become increasingly prevalent. Compliance in taking medication is not 100% for a number of reasons. This would lead to ASIH and potentially adverse events. A clinical situation would be elevation of LFTs (liver function tests) and impending liver dysfunction. Pens et. al. was a clear example of the adverse consequences with AAS cessation. AAS cessation was required in the treatment of polycythemia brought upon by continuous AAS administration.[]
 A medical quandary for many physicians presented with hypogonadal patients, standard treatment to this point has been testosterone replacement therapy, human chorionic gonadotropin (hCG), or conservative therapy (i.e. nothing). The primary drawback of testosterone replacement is that this therapy is infinite in nature. Exogenous testosterone serves only to remedy the symptoms of suppressed testicular/gonadotropin production. While it may transiently combat the lean muscle atrophy, declining muscular strength, decreased libido, erection dysfunction, and depression associated with hypogonadism, it will not stimulate endogenous testosterone production. Administered testosterone will only suppress testicular function further.
A medical quandary for many physicians presented with hypogonadal patients, standard treatment to this point has been testosterone replacement therapy, human chorionic gonadotropin (hCG), or conservative therapy (i.e. nothing). The primary drawback of testosterone replacement is that this therapy is infinite in nature. Exogenous testosterone serves only to remedy the symptoms of suppressed testicular/gonadotropin production. While it may transiently combat the lean muscle atrophy, declining muscular strength, decreased libido, erection dysfunction, and depression associated with hypogonadism, it will not stimulate endogenous testosterone production. Administered testosterone will only suppress testicular function further.
It is important to understand that the use of a treatment for HPTA restoration at this time would only be effective in those individuals who had a normal HPTA functionality prior to AAS administration. This is not to say that there may be developed something in the future that will be effective for other causes of HPTA dysfunction. The regulation of the HPTA is an active area of investigation. There are other factors that interact with the HPTA which may show promise in their ability to restore HPTA health. The influences of other hormones within the endocrine system and the HPTA have only partially been explored.
The normal operation of both the testicular and hypothalamic-pituitary regions is crucial in returning HPTA function to normal. Returning one component of the axis to normal without concurrently returning the other would sabotage and inhibit the operation of the entire HPTA. The ability to produce a cure whereby there is no longer a need for medication is small. Discounting costs and focusing strictly on medicine reasons for this include inadequate stimulation for a critical part of the HPTA for full restoration, secondary inhibition of the HPTA, inadequate follow up and monitoring, and compliance due to the length of time the medicines are prescribed.
HISTORY
History has not been kind to AAS users whether illicit or prescribed. Undoubtedly, heavy politicization of AAS, constant media and press coverage, and the total failure of the medical community to properly investigate this class of medications have lead to ignorance among the public and professional, alike. The hysteria surrounding AAS is unprecedented as demonstrated by the draconian measures the government has applied to illicit AAS users. A considerable amount of the fault lies at the door of the medical profession who has capitulated lock, stock, and barrel to the pundits who barely are able to pronounce AAS never mind name on other than testosterone.
But what is the most horrific in the history of AAS is the mind warp that the medical/ research establishment took after 1995. While finally admitting that there is a positive relationship between androgens and muscle the medical community has managed at the same time to have sentenced countless individuals to harm. One would have to been blind, deaf, and dumb and possibly dead to not recognize the relationship between androgens ands muscle. Apparently, the medical community was in a coma. The observational idea from association between androgens and muscle, of course, came from bodybuilders. Had any of the “white coats” ever come down from their Ivory Towers and bothered to ask the bodybuilders they would have been told about ASIH. It would not have been called that but there is no doubt they would have been told of post cycle signs and symptoms. But they did not ask, decided to ignore the principles of life, and in turn revealed once again their ability to make mistakes on a grand scale. Below is a summary of AAS history and the beliefs held by the athletic and bodybuilder community, academic/ physician, and what research ahs shown.
|
Beliefs Held by the Athletic and the Academic Communities On AAS
|
||
|
BodyBuilders Beliefs held by recreational bodybuilders and athletic community. |
Research What has been demonstrated.. |
Physicians/Academics The physician/ academic view and belief. |
|
AAS increase muscle mass, strength, and athletic performance. |
Replacement doses of testosterone when administered to hypogonadal men and supraphysiological doses when administered to eugonadal men increase fat-free mass, muscle size, and strength. |
Only replacement doses of testosterone when given to hypogonadal men and prepubertal boys have anabolic effects. Supra- physiological doses of testosterone do not further increase muscle mass. |
|
Higher doses of AAS promote greater increases in muscle mass and strength than lower doses; administering more than one androgenic steroid simultaneously (stacking) produces greater increases in muscle mass and strength than any single agent alone. |
A linear dose–response relationship exists between testosterone dose and its anabolic effects over a wide range of concentrations extending from subphysiologic to supraphysiologic range. |
Beyond the physiologic range, further increases in the dose of AAS would produce no further gains in fat-free mass and muscle strength. |
|
The anabolic and androgenic activities of AAS can be dissociated, so that some derivatives of testosterone have preferentially greater anabolic activity than androgenic activity. |
Different androgen- dependent processes have different dose– response relationships. |
The anabolic and androgenic activity cannot be dissociated; they are described by the same dose–response relationship. |
|
The anabolic and androgenic effects are mediated through separate mechanisms and thus can be dissociated. |
The anabolic effects are likely mediated through an androgen-receptor- mediated mechanism that involves recruitment of tissue- specific coactivators and corepressors. |
The anabolic effects are mediated through an androgen-receptor- mediated mechanism. |
|
The effects of AAS administration cause an up-regulation of the skeletal muscle androgen receptor (AR). |
AAS administration causes a upregulation of the skeletal muscle and bone androgen receptor (AR). |
The effects of AAS administration cause a down-regulation of the skeletal muscle androgen receptor (AR). |
|
HPTA Normalization after AAS cessation is variable and sometimes may never occur. |
The severity and duration of ASIH after AAS cessation is unknown and has been reported to take over 2+years. |
AAS cessation uniformly results in HPTA normalization within 2 weeks to several months. |
|
Signs & symptoms after AAS cessation are due to inadequate gonadal function. |
There is no medical or scientific literature that supports AAS dependency/ addiction. AAS dependency/addiction is not a recognized disease within the ICD-10 or the DSM-IV. |
AAS use is associated with adverse health consequences that include chemical dependency/addiction. |
FUTURE DIRECTIONS
It is time for the medical community to act responsibly, intelligently, and forcefully and take control of the medical care for individuals. At the very minimum the
I. Uniform definition and diagnosis of ASIH.
II. Investigations on a more accurate estimate of ASIH prevalence.
III. A does-response study on AAS and HPTA normalization. Clinical investigations regarding AAS (type, dose, duration, etc) to development of ASIH (severity of signs & symptoms, duration, HPTA normalization).
IV. Clinical investigations on medical treatments (prevent, eliminate, or minimize) for ASIH.
V. Investigations on the development of protocols or programs to effect positive body composition changes without the attendant consequences of ASIH.
VI. Collaborative clinical investigations regarding dependence, abuse, and addiction of androgens in relation to ASIH.
References
[1] Government Reform Committee Hearing, United States House of Representatives, on March 17, 2005. “Restoring Faith in America’s Pastime: Evaluating Major League Baseball’s Efforts to Eradicate Steroid Use.” One Hundred Ninth Congress, First Session. Available via the World Wide Web: http://www.gpo.gov/congress/house ; http://www.house.gov/reform
[2] Urhausen, A., Torsten, A., & Wilfried, K. (2003). Reversibility of the effects on blood cells, lipids, liver function and hormones in former anabolic-androgenic steroid abusers. J Steroid Biochem Mol Biol, 84(2-3), 369-375. van Breda, E., Keizer, H. A., Kuipers, H., & Wolffenbuttel, B. H. (2003). Androgenic anabolic steroid use and severe hypothalamic-pituitary dysfunction: a case study. Int J Sports Med, 24(3), 195-196.
[3] Pena, J. E., Thornton, M. H., Jr., & Sauer, M. V. (2003). Reversible azoospermia: anabolic steroids may profoundly affect human immunodeficiency virus-seropositive men undergoing assisted reproduction. Obstet Gynecol, 101(5 Pt 2), 1073-1075.
[4] Bhasin et al., (1996), The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men, NEJM 335(1): 1-7.
[5] Knuth, U. A., H. Maniera, et al. (1989). “Anabolic steroids and semen parameters in bodybuilders.” Fertil Steril 52(6): 1041-7.
[6] See FN1. Kirk J. Brower, M.D., University of Michigan.
[7] TX LEGIS 1177 (2005), 2005 Tex. Sess. Law Serv. Ch. 1177 (H.B. 3563) (VERNON’S).
[8] Tenover JS. Effects of Testosterone Supplementation in the Aging Male. Journal of Clinical Endocrinology and Metabolism. 1992; 75: 1092-1098. Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The Effects of Supraphysiologic Doses of Testosterone on Muscle Size and Strength in Normal Men. New England Journal of Medicine. 1996 July 4; 335: 1-7. Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R. Testosterone Replacement Increases Fat-Free Mass and Muscle Size in Hypogonadal Men. Journal of Clinical Endocrinology and Metabolism. 1997; 82(2): 407-413. Hervey GR, Knibbs AV, Burkinshaw L, Morgan DB, Jones PRM, Chettle DR, Vartsky D. Effects of Methandienone on the Performance and Body Composition of Men Undergoing Athletic Training. Clinical Science. 1981; 60(4): 457-461.
[9] Schiavi RC, Schreiner-Engel P, White D, Mandeli J. The Relationship Between Pituitary-Gonadal Function and Sexual Behavior in Healthy Aging Men. Psychosomatic Medicine. 1991 Jul-Aug; 53 (4): 363-374.
[10] See FN4. Bhasin et al, 1996; 1997; Hervey et al, 1981; See FN10. Sih R, Morley JE, Kaiser FE, Perry III HM, Patrick P, Ross C. Testosterone Replacement in Older Hypogonadal Men: a 12-Month Randomized Controlled Trial. Journal of Clinical Endocrinology and Metabolism. 1997; 82: 1661-1667.
[11] See FN8.. Tenover, 1992; See FN10. Sih et al, 1997; Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R. Testosterone Replacement Increases Fat-Free Mass and Muscle Size in Hypogonadal Men. Journal of Clinical Endocrinology and Metabolism 1997; 82(2): 407-413. Evans RP and Amerson AB. 1974 Androgens and Erythropoiesis. Journal of Clinical Pharmacology. 1974; 14: 94-101.
[12] See FN8. Tenover, 1992; Anderson et al, 1996; 1997; Baran DT, Bergfeld MA, Teitelbaum SL, Avioli LV. Effect of Testosterone Therapy on Bone Formation in an Osteoporotic Hypogonadal Male. Calcified Tissue Research. 1978 Dec; 26(2): 103-106.
[13] Alexander GM, Swerdloff RS, Wang C, Davidson T, McDonald V, Steiner B, Hines M. April Androgen-behavior Correlations in Hypogonadal Men and Eugonadal Men. II. Cognitive Abilities. Hormones and Behavior. 1998; 33(2): 85-94.
[14] Starr C, Taggart R. Integration and Contol: Endocrine Systems. In: Star C, Taggart R, eds. Biology-The Unity and Diversity of Life. Belmont, California: Wadsworth Publishing Company,1992: 587-590.
[15] Rabkin JG, Wagner GJ, Rabkin R. Testosterone Therapy for Human Immunodeficiency Virus-Positive Men With and Without Hypogonadism. Journal of Clinical Psychopharmacology. 1999 Feb; 19(1): 19-27. Rabkin JG, Wagner GJ, Rabkin R. A Double-Blind, Placebo-Controlled Trial of Testosterone Therapy for HIV-Positive Men With Hypogonadal Symptoms. Archives of General Psychiatry. 2000 Feb; 57(2): 141-147. Sattler FR, Jaque SV, Schroeder ET, Olson C, Dube MP, Martinez C, Briggs W, Horton R, Azen S. Effects of Pharmacological Doses of Nandrolone Decanoate and Progressive Resistance Training in Immunodeficient Patients Infected with Human Immunodeficiency Virus. Journal of Clinical Endocrinology and Metabolism. 1999; 84(4): 1268-1276. Strawford A, Barbieri T, Neese R, Van Loan M, Christiansen M, Hoh R, Sathyan G, Skowronski R, King J, Hellerstein M. Effects of Nandrolone Decanoate Therapy in Borderline Hypogonadal Men With HIV-Associated Weight Loss. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1999 Feb 1; 20(2): 137-146. Strawford A, Barbieri T, Van Loan M, Parks E, Catlin D, Barton N, Neese R, Christiansen M, King J, Hellerstein MK. Resistance Exercise and Supraphysiologic Androgen Therapy in Eugonadal Men With HIV-Related Weight Loss: a Randomized Controlled Trial. JAMA. 1999 April 14; 281(14): 1282-1290. Grinspoon C, Corcoran C, Askari H, Schoenfeld D, Wolf L, Burrows B, Walsh M, Hayden D, Parlman K, Anderson E, Basgoz N, Klibanski A. Effects of Androgen Administration in Men With the AIDS Wasting Syndrome. A Randomized, Double-Blind, Placebo-Controled Trial. Annals of Internal Medicine. 1998 July 1; 129(1): 18-26. Grinspoon S, Corcoran C, Anderson E, Hubbard J, Stanley T, Basgoz N, Klibanski A. Sustained Anabolic Effects of Long-Term Androgen Administration in Men With AIDS Wasting. Clinical Infectious Diseases. 1999 Mar; 28(3): 634-636. Grinspoon S, Corcoran C, Parlman K, Costello M, Rosenthal D, Anderson E, Stanley T, Schoenfeld D, Burrows B, Hayden D, Basgoz N, Klibanski A. Effects of Testosterone and Progressive Resistance Training in Eugonadal Men With AIDS Wasting. A Randomized, Controlled Trial. Annals of Internal Medicine. 2000 Sep 5; 133(5): 348-355. Grinspoon S, Corcoran C, Stanley T, Baaj A, Basgoz N, Klibanski A. Effects of Hypogonadism and Testosterone Administration on Depression Indices in HIV-Infected Men. Journal of Clinical Endocrinology and Metabolism. 2000 Jan; 85(1): 60-5. Van Loan MD, Strawford A, Jacob M, Hellerstein M. Monitoring Changes in Fat-Free Mass in HIV-Positive Men With Hypotestosteronemia and AIDS Wasting Syndrome Treated With Gonadal Hormone Replacement Therapy. AIDS. 1999 Feb 4; 13(2): 241-248. Bhasin S, Storer TW, Javanbakht M, Berman N, Yarasheski KE, Phillips J, Dike M, Sinha-Hikim I, Shen R, Hays RD, Beall G. Testosterone Replacement and Resistance Exercise in HIV-Infected Men With Weight Loss and Low Testosterone Levels. JAMA. 2000 Feb 9; 283(6): 763-770.
[16] See FN8. Tenover, 1992; See FN11. Bhasin et al, 1997; Sih et al, 1997; See FN15. Rabkin et al, 1999; Wagner GJ, Rabkin JG. Testosterone Therapy for Clinical Symptoms of Hypogonadism in Eugonadal Men With AIDS. International Journal of STD and AIDS. 1998 Jan; 9(1): 41-44. Wang C, Swedloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N. Transdermal Testosterone Gel Improves Sexual Function, Mood, Muscle Strength, and Body Composition Parameters in Hypogonadal Men. Testosterone Gel Study Group. Journal of Clinical Endocrinology and Metabolism. 2000 Aug; 85(8): 2839-2853. Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A, Santanna J, Loh L, Lenrow DA, Holmes JH, Kapoor SC, Atkinson LE, Strom BL. Effects of Testosterone Replacement in Hypogonadal Men. Journal of Clinical Endocrinology and Metabolism. 2000 Aug; 85(8): 2670-2677. Davidson JM, Camargo CA, Smith ER. Effects of Androgen on Sexual Behavior in Hypogonadal Men. Journal of Clinical Endocrinology and Metabolism. 1979 Jun; 48(6): 955-958.
[17] Rakic Z, Starcevic V, Starcevic VP, Marinkovic J. Testosterone Treatment in Men with Erectile Disorder and Low Levels of Total Testosterone in Serum. Archives of Sexual Behavior. 1997 Oct; 26(5): 495-504. Lawrence IG, Price DE, Howlett TA, Harris KP, Feehally J, Walls J. Correcting Impotence in the Male Dialysis Patient: Experience With Testosterone Replacement and Vacuum Tumescence Therapy. American Journal of Kidney Disorders. 1998 Feb; 31(2): 313-319. Morales A, Johnston B, Heaton JP, Lundie M. Testosterone Supplementation for Hypogonadal Impotence: Assessment of Biochemical Measures and Therapeutic Outcomes. Journal of Urology. 1997 Mar; 157(3): 849-854. Morales A, Johnston B, Heaton JW, Clark A. Oral Androgens in the Treatment of Hypogonadal Impotent Men. Journal of Urology. 1994 Oct; 152(4): 115-1118. McClure RD, Oses R, Ernest ML. Mar Hypogonadal Impotence Treated by Transdermal Testosterone. Urology. 1991; 37(3): 224-228. Carani C, Zini D, Baldini A, Della Casa L, Ghizzani A, Marrama P. Effects of Androgen Treatment in Impotent Men with Normal and Low Levels of Free Testosterone. Archives of Sexual Behavior. 1990 Jun; 19(3): 223-234. Carey PO, Howards SS, Vance ML. Transdermal Testosterone Treatment of Hypogonadal Men. Journal of Urology. 1988 Jul; 140(1): 76-79. Nankin HR, Lin T, Osterman J. Chronic Testosterone Cypionate Therapy in Men with Secondary Impotence. Fertility and Sterility. 1986 Aug; 46(2): 300-307. Klepsch I, Maicanescu-Georgescu M, Marinescu L. Clinical and Hormonal Effects of Testosterone Undecanoate (TU) in Male Sexual Impotence. Endocrinologie. 1982 Oct-Dec; 20(4): 289-293. Schiavi RC, White D, Mandeli J, Levine AC. Effect of Testosterone Administration on Sexual Behavior and Mood in Men with Erectile Dysfunction. Archives of Sexual Behavior. 1997 Jun; 26 (3): 231-241.
[18] Demling RH, DeSanti L. Oxandrolone, an Anabolic Steroid, Significantly Increases the Rate of Weight Gain in the Recovery Phase After Major Burns. The Journal of Trauma. 1997 Jul; 43(1): 47-51.
[19] Gascon A, Belvis JJ, Berisa F, Iglesias E, Estopinan V, Terul JL. Nandrolone Decanoate is a Good Alternative for the Treatment of Anemia in Elderly Male Patients on Hemodialysis. Geriatric Nephrol Urol. 1999; 9(2): 67-72. Hurtado R, Sosa R, Majluf A, Labardini JR. Refractory Anaemia (RA) Type I FAB Treated With Oxymetholone (OXY): Long-Term Results. British Journal of Haematology. 1993 Sep; 85(1): 235-236. Doney K, Pepe M, Storb R, Bryant E, Anasetti C, Appelbaum FR, Buckner CD, Sanders J, Singer J, Sullivan K, et al. Immunosuppressive Therapy of Aplastic Anemia: Results of a Prospective, Randomized Trial of Antithymocyte Globulin (ATG), Methylprednisolone, and Oxymetholone to ATG, Very High-Dose Methylprednisolone, and Oxymetholone. Blood. 1992 May 15; 79(10): 2566-2571. Stricker RB and Shuman MA. Aplastic Anaemia Complicating Systemic Lupus Erythematosus: Response to Androgens in Two Patients. American Journal of Hematology. 1984 Aug; 17(2): 193-201.
[20] Tomoda H. Effect of Oxymetholone on Left Ventricular Dimensions in Heart Failure Secondary to Idiopathic Dilated Cardiomyopathy or to Mitral or Aortic Regurgitation. American Journal of Cardiology. 1999 Jan 1; 83(1): 123-5.
[21] Hobbs CJ, Jones RE, Plymate SR. Nandrolone, a 19-Nortestosterone, Enhances Insulin-Independent Glucose Uptake in Normal Men. Journal of Clinical Endocrinology and Metabolism. 1996 Apr; 81(4): 1582-1585.
[22] Dombros NV, Digenis GE, Soliman G, Oreopoulos DG. Anabolic Steroids in the Treatment of Malnourished CAPD Patients: a Retrospective Study. Peritoneal Dialysis International. 1994; 14(4): 344-347.
[23] Bonkovsky HL, Singh RH, Jafri IH, Fiellin DA, Smith GS, Simon D, Cotsonis GA, Slaker DP. A Randomized, Controlled Trial of Treatment of Alcoholic Hepatitis with Parental Nutrition and Oxandrolone. II. Short-term Effects on Nitrogen Metabolism, Metabolic Balance, and Nutrition. American Journal of Gastroenterology. 1991 Sep; 86(9): 1209-1218. Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, Schiff ER, McClain CJ, Marsano LS, Allen JI. A Study of Oral Nutritional Support with Oxandrolone in Malnourished Patients with Alcoholic Hepatitis: Results of a Department of Veterans Affairs Cooperative Study. Hepatology. 1993 April; 17(4): 564-576.
[24] Kley HK, Stremmel W, Kley JB, Schaghecke R. Testosterone Treatment of Men With Idiopathic Hemochromatosis. Clinical Investigation. 1992 Jul; 70(7): 566-572.
[25] Anderson FH, Francis RM, Faulkner K. Androgen Supplementation in Eugonadal Men with Osteoporosis: Effects of Six Months of Treatment on Bone Mineral Density and Cardiovascular Risk Factors. Bone. 1996 Feb; 18(2): 171-177. Anderson FH, Francis RM, Peaston RT, Wastell HJ. Androgen Supplementation in Eugonadal Men With Osteoporosis: Effects of Six Months’ Treatment on Markers of Bone Formation and Resorption. Journal of Bone and Mineral Research. 1997 Mar;12(3): 472-478. Prakasam G, Yeh JK, Chen MM, Castro-Magana M, Liang CT, Aloia JF. Effects of Growth Hormone and Testosterone on Cortical Bone Formation and Bone Density in Aged Orchiectomized Rats. Bone. 1999 May; 24(5): 491-497. (Anderson et al, 1996; 1997; Baran et al, 1978; Hamdy RC, Moore SW, Whalen KE, Landy C. Nandrolone Decanoate for Men with Osteoporosis. American Journal of Therapeutics. 1998 Mar; 5(2): 89-95. Behre HM, Kliesch S, Leifke E, Link TM, Nieschlag E. Long-Term Effect of Testosterone Therapy on Bone Mineral Density in Hypogonadal Men. Journal of Clinical Endocrinology and Metabolism. 1997 Aug; 82(8): 2386-2390.
[26] Alen M, Rahkila P, Reinila M, Vihko R. Androgenic-Anabolic Steroid Effects on Serum Thyroid, Pituitary and Steroid Hormones in Athletes. American Journal of Sports Medicine. 1987; 15: 357-361. Bijlsma JWJ, Duursma SA, Thijssen JHH, Huber O. Influence of Nandrolondecanoate on the Pituitary-Gonadal Axis in Males. Acta Endocrinologica. 1982 Sep; 101: 108-112. See FN4. Bhasin et al, 1996; See FN15. Strawford et al, 1999; Clerico A, Ferdeghini M, Palombo C, Leoncini R, Del Chicca MG, Sardano G, Mariani G. Effect of Anabolic Treatment on the Serum Levels of Gonadotropins, Testosterone, Prolactin, Thyroid Hormones and Myoglobin of Male Athletes Under Physical Training. Journal of Nuclear Medicine and Allied Science. 1981 July-Sep; 25(3): 79-88. Stromme SB, Meen HD, Aakvaag A. Effects of an Androgenic-Anabolic Steroid on Strength Development and Plasma Testosterone Levels in Normal Males. Medicine and Science in Sports and Exercise. 1974; 6: 203-208.
[27] Clerico A, Ferdeghini M, Palombo C, Leoncini R, Del Chicca MG, Sardano G, Mariani G. Effect of Anabolic Treatment on the Serum Levels of Gonadotropins, Testosterone, Prolactin, Thyroid Hormones and Myoglobin of Male Athletes Under Physical Training. Journal of Nuclear Medicine and Allied Science 1981 July-Sep; 25(3): 79-88.
[28] Marynick SP, Loriaux DL, Sherins RJ, Pita JC Jr, Lipsett MB. 1979 Sep Evidence that Testosterone can Suppress Pituitary Gonadotropin Secretion Independently of Peripheral Aromatization. Journal of Clinical Endocrinology and Metabolism. 49(3): 396-398. See FN8. Tenover, 1992; See FN4. Bhasin et al, 1996; See FN15. See FN15. Strawford et al, 1999;
[29] Jarow JP and Lipshultz LI. Anabolic Steroid-Induced Hypogonadotropic Hypogonadism. American Journal of Sports Medicine. 1990 Jul-Aug; 18(4): 429-431.
[30] See FN4. Bhasin et al, 1996.
[31] See FN15. Sattler et al, 1999.
[32] Bagatell CJ, Matsumoto AM, Christensen RB, Rivier JE, Bremner WJ. Comparison of a gonadotropin releasing –hormone antagonist plus testosterone (T) versus T alone as potential male contraceptive regimens. Journal of Clinical Endocrinology and Metabolism. 1993 Aug; 77(2): 427-32.
[33] Bagatell CJ, Heiman JR, Matsumoto AM, Rivier JE, Bremner WJ. Metabolic and Behavioral Effects of High-Dose, Exogenous Testosterone in Healthy Men. Journal of Clinical Endocrinology and Metabolism. 1994 Aug; 79(2): 561-567. Tricker R, Casaburi R, Storer TW, Clevenger B, Berman N, Shirazi A, Bhasin S. The Effects of Supraphysiological Doses of Testosterone on Angry Behavior in Healthy Eugonadal Men- A Clinical Research Center Study. Journal of Clinical Endocrinology and Metabolism. 1996 Oct; 81(10): 3754-3758. See FN58. Sheffield-Moore et al, 1999; See FN15. Sattler et al, 1999;See FN25. Behre et al, 1997.
[34] Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, Veldhuis JD, Urban RJ. Testosterone Deficiency in Young Men: Marked Alterations in Whole Body Protein Kinetics, Strength, and Adiposity. Journal of Clinical Endocrinology and Metabolism. 1998; 83: 1886-1892.
[35] See FN9.
[36] Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of Aging on In Vivo Synthesis of Skeletal Muscle Myosin Heavy-Chain and Sarcoplasmic Protein in Humans. American Journal of Physiology. 1997; 273 (4 pt 1): E790-800. See FN34. Mauras et al, 1998;
[37] See FN17. Rakic et al, 1997.
[38] Vermeulen A, Kaufman JM. Ageing of the Hypothalamo-Pituitary-Testicular Axis in Men. Hormonal Research. 1995; 43 (1-3): 25-28.
[39] See FN34. Mauras et al, 1998.
[40] Wishart JM, Need AG, Horowitz M, Morris HA, Nordin BE. Effect of Age on Bone Density and Bone Turnover in Men. Clinical Endocrinology. 1995; 42: 141-146.
[41] Bijlsma JW, Duursma SA, Thijssen JH, Huber O, (1982), Influence of nandrolondecanoate on the pituitary-gonadal axis in males, Acta Endocrinol (Copenh). Sep; 101(1): 108-12.
[42] Maeda Y, Nakanishi T, Ozawa K, Kijima Y, Nakayama I, Shoji T, Sasaoka T, (1989), Anabolic steroid-associated hypogonadism in male hemodialysis patients, Clin Nephrol. Oct; 32(4): 198-201.
[43] Forbes, G. B., Porta, C. R., Herr, B. E., & Griggs, R. C. (1992). Sequence of changes in body composition induced by testosterone and reversal of changes after drug is stopped. JAMA, 267(3), 397-399.
[44] Bhasin et al., (1996), The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men, NEJM 335(1): 1-7.
[45] Sattler et al 1999. Effects of pharmacological doses of nandrolone decanoate and progressive resistance training in immunodeficient patients infected with human immunodeficiency virus. J Clin Endocrinol Metab. Apr;84(4):1268-76; See FN15. Strawford A et al 1999. Effects of nandrolone decanoate therapy in borderline hypogonadal men with HIV-associated weight loss. Acquir Immune Defic Syndr Hum Retrovirol. Feb 1;20(2):137-46.
[46] Navarro JF et al., (1998), Androgens for the treatment of anemia in peritoneal dialysis patients, Adv Perit Dial.; 14: 232-5.
[47] Gascon A et al., (1999), Nandrolone decanoate is a good alternative for the treatment of anemia in elderly male patients on hemodialysis, Geriatr Nephrol Urol.; 9(2): 67-72.
[48] Johansen KL, Mulligan K, Schambelan M, (1999), Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial, JAMA. 1999 Apr 14; 281(14): 1275-81.
[49] Pena JE, Thornton MH, Jr., & Sauer MV, (2003), Reversible azoospermia: anabolic steroids may profoundly affect human immunodeficiency virus-seropositive men undergoing assisted reproduction. Obstet Gynecol, 101(5 Pt 2), 1073-1075.
[50] See FN2.
[51] See FN2.
[52] Schroeder, E. T., Singh, A., Bhasin, S., Storer, T. W., Azen, C., Davidson, T., et al. (2003). Effects of an oral androgen on muscle and metabolism in older, community-dwelling men. Am J Physiol Endocrinol Metab, 284(1), E120-128.
[53] Complaint to OHRP by Michael C. Scally, M.D. 16 June 2003.
[54] Okuyama A, Nakamura M, Namiki M, Aono T, Matsumoto K, Utsunomiya M, Yoshioka T, Itoh H, Itatani H, Mizutani S, et al. Testicular Responsiveness to Long-Term Administration of hCG and HMG in Patients with Hypogonadotrophic Hypogonadism. Hormone Research. 1986; 23(1): 21-30. Cisternino M, Manzoni SM, Coslovich E, Autelli M. Hormonal Replacement Therapy with hCG and HU-FSH in Thalassaemic Patients Affected by Hypogonadotropic Hypogonadism. Journal of Pediatric Endocrinology and Metabolism. 1998; 11 Suppl 3: 885-890. Burgess S, Calderon MD. Subcutaneous Self-Administration of Highly Purified Follicle Stimulating Hormone and Human Chorionic Gonadotrophin for the Treatment of Male Hypogonadotrophic Hypogonadism. Spanish Collaborative Group on Male Hypogonadotropic Hypogonadism. Human Reproduction. 1997 May; 12(5): 980-986. Martikainen H, Alen M, Rahkila P, Vihko R. Testicular Responsiveness to Human Chorionic Gonadotrophin During Transient Hypogonadotrophic Hypogonadism Induced by Androgenic/Anabolic Steroids in Power Athletes. Journal of Steroid Biochemistry. 1986 July; 25(1): 109-112. Barrio R, de Luis D, Alonso M, Lamas A, Moreno JC. Induction of Puberty with Human Chorionic Gonadotropin and Follicle-Stimulating Hormone in Adolescent Males With Hypogonadotrophic Hypogonadism. Fertility and Sterility. 1999 Feb; 71(2): 244-248. D’Agata R, Heindel JJ, Vicari E, Aliffi A, Gulizia S, Polosa P. hCG-Induced Maturation of the Seminiferous Epithelium in Hypogonadotropic Men. Hormone Research. 1984; 19(1): 23-32. D’Agata R, Vicari E, Aliffi A, Maugeri G, Mongioi A, Gulizia S. Testicular Responsiveness to Chronic Human Chorionic Gonadotropin Administration in Hypogonadotropic Hypogonadism. Journal of Clinical Endocrinology and Metabolism. 1982 Jul; 55(1): 76-80. Burgess et al, 1997; Vicari E, Mongioi A, Calogero AE, Moncada ML, Sidoti G, Polosa P, D’Agata R. Therapy With Human Chorionic Gonadotrophin Alone Induces Spermatogenesis in Men With Isolated Hypogonadotrophic Hypogonadism- Long-Term Follow-Up. International Journal of Andrology. 1992 Aug; 15(4): 320-329. Ulloa-Aguirre A, Mendez JP, Diaz-Sanchez V, Altamirano A, Perez-Palacios G. Self-priming Effect of Luteinizing Hormone-Human Chorionic Gonadotropin (hCG) Upon the Biphasic Testicular Response to Exogenous hCG. I. Serum Testosterone Profile. Journal of Clinical Endocrinology and Metabolism. 1985 Nov; 61(5): 926-932. Liu L, Banks SM, Barnes KM, Sherins RJ. Two-year Comparison of Testicular Responses to Pulsatile Gonadotropin-Releasing Hormone and Exogenous Gonadotropins from the Inception of Therapy in Men with Isolated Hypogonadotropic Hypogonadism. Journal of Clinical Endocrinology and Metabolism. 1988 Dec; 67(6): 1140-1145. Ley SB, Leonard JM. Male Hypogonadotropic Hypogonadism: Factors Influencing Response to Human Chorionic Gonadotropin and Human Menopausal Gonadotropin, Including Prior Exogenous Androgens. Journal of Clinical Endocrinology and Metabolism. 1985 Oct; 61(4): 746-752. Kelly WF, Kjeld JM, Mashiter K, Joplin GF. Reassessment of the Human Chorionic Gonadotropin Stimulation Test in Hypogonadal Males. Archives of Andrology. 1982 Feb; 8(1): 53-59. Dunkel L, Perheentupa J, Sorva R. Single versus Repeated Dose Human Chorionic Gonadotropin Stimulation in the Differential Diagnosis of Hypogonadotropic Hypogonadism. Journal of Clinical Endocrinology and Metabolism. 1985 Feb; 60(2): 333-337.
[55] See FN54. Burgess S and Calderon MD, 1997.
[56] Menon, D. K. (2003). Successful treatment of anabolic steroid-induced azoospermia with human chorionic gonadotropin and human menopausal gonadotropin. Fertil Steril, 79 Suppl 3, 141-143.
[57] Sheikholislan BM & Stempefel RS. Hereditary isolated somatotropin deficiency: effects of human growth hormone administration. Pediatrics 1972 49 362–374. Paulsen CA, Espeland DH & Michail EE. Effects of HCG, hMG, hLH and GH administration on testicular function. In Human Testis, pp 547–562. Eds E Rosemberg & CA Paulsen. New York: Plenum Press, 1970. Balducci R, Toscano V, Mangiantini A, Bianchi P, Guglielmi R & Boscherini B. The effect of growth hormone administration on testicular response during gonadotropin therapy in subjects with combined gonadotropin and growth hormone deficiencies. Acta Endocrinologica 1993 128 19–23. Chatelain PG, Sanchez P & Saez JM. Growth hormone and insulin-like growth factor-I treatment increase testicular luteinizing hormone receptors and steroidogenic responsiveness of growth hormone deficient dwarf mice. Endocrinology 1991 128 1857–1862.
[58] See FN54. Balducci R 1993.
[59] Carani C, Granata AR, De Rosa M, Garau C, Zarrilli S, Paesano L, Colao A, Marrama P, Lombardi G. The effect of chronic treatment with GH on gonadal function in men with isolated GH deficiency. Eur J Endocrinol. 1999 Mar;140(3):224-30.
[60] Bjork JT, Varma RR, Borkowf HI. Clomiphene Citrate Therapy in a Patient with Laennec’s Cirrhosis. Gastroenterology. 1977 Jun; 72(6): 1308-1311. Landefeld CS, Schambelan M, Kaplan SL, Embury SH. Clomiphene-Responsive Hypogonadism in Sickle Cell Anemia. Annals of Internal Medicine. 1983 Oct; 99(4): 480-483. Spijkstra JJ, Spinder T, Gooren L, van Kessel H. Divergent Effects of the Antiestrogen Tamoxifen and of Estrogens on Luteinizing Hormone (LH) Pulse Frequency, but not on Basal LH Levels and LH Pulse Amplitude in Men. Journal of Clinical Endocrinology and Metabolism. 1988 Feb; 66(2): 355-360. Lim VS, Fang VS. Restoration of Plasma Testosterone Levels in Uremic Men With Clomiphene Citrate. Journal of Clinical Endocrinology and Metabolism. 1976 Dec; 43(6): 1370-1377.
Ross LS, Kandel GL, Prinz LM, Auletta F. Clomiphene Treatment of the Idiopathic Hypofertile Male: High-Dose, Alternate-Day Therapy. Fertility and Sterility. 1980 Jun; 33(6): 618-623. Guay AT, Bansal S, Heatley GJ. Effect of Raising Endogenous Testosterone Levels in Impotent Men With Secondary Hypogonadism: Double Blind Placebo-Controlled Trial with Clomiphene Citrate. Journal of Clinical Endocrinology and Metabolism. 1995 Dec; 80(12): 3546-3552. Burge MR, Lanzi RA, Skarda ST, Eaton RP. Idiopathic Hypogonadotropic Hypogonadism in a Male Runner is Reversed by Clomiphene Citrate. Fertility and Sterility. 1997 April; 67(4): 783-785. Ross et al, 1980;
[61] Tan, R. S., & Vasudevan, D. (2003), Use of clomiphene citrate to reverse premature andropause secondary to steroid abuse, Fertil Steril, 79(1), 203-205.
[62] Guay, A. T., Jacobson, J., Perez, J. B., Hodge, M. B., & Velasquez, E. (2003), Clomiphene increases free testosterone levels in men with both secondary hypogonadism and erectile dysfunction: who does and does not benefit? Int J Impot Res, 15(3), 156-165.
[63] Krause W, Hubner HM, Wichmann U. Treatment of Oligozoospermia by Tamoxifen: No Evidence for Direct Testicular Action. Andrologia. 1985 May-June; 17(3): 285-290. Lewis-Jones DI, Lynch RV, Machin DC, Desmond AD. Improvement in Semen Quality in Infertile Males After Treatment with Tamoxifen. Andrologia. 1987 Jan-Feb; 19(1): 86-90. Gazvani MR, Buckett W, Luckas MJM, Aird IA, Hipkin LJ, Lewis-Jones DI. Conservative management of azoospermia following steroid abuse. Human Reproduction. 1997; 12(8): 1706-1708. Wu FCW, Farley TMM, Peregoudov A, Waites GMH. Effects of testosterone enanthate in normal men: experience from a multicenter contraceptive efficacy study. Fertility and Sterility. 1996 Mar; 65(3): 626-636.
[64] Sheffield-Moore M, Urban RJ, Wolf SE, Jiang J, Catlin DH, Herndon DN, Wolfe RR, Ferrando AA. Short-term Oxandrolone Administration Stimulates Net Muscle Protein Synthesis in Young Men. Journal of Clinical Endocrinology and Metabolism. 1999; 84: 2705-2711. Shelton DL. 2000 Aug 7 Testosterone Therapy Hype May Be Creating False Hopes. http://www.ama-assn.org/sci-pubs/amnews/pick_oo/hll20807.htm Lacayo R. Are You Man Enough? Time Magazine. 2000 April 24; 155: 58-64.
[65] See FN58. Shelton DL, 2000.
[66] Centers for Disease Control. Division of HIV/AIDS Prevention. Survey Report vol. 12, No. 2. Table 6. www.cdc.gov. Centers for Disease Control. Division of HIV/AIDS Prevention. Survey Report vol. 12, No. 2. Table 5. www.cdc.gov.
[67] Centers for Disease Control. Division of HIV/AIDS Prevention. Survey Report vol. 12, No. 2. Figure 3. www.cdc.gov
[68] See FN8. Tenover, 1992; See FN11. Bhasin et al, 1997; See FN10. Sih et al, 1997; See FN15. Rabkin et al, 1999; See FN16. Wagner & Rabkin 1998; See FN16. Wang et al, 2000; See FN16. Snyder et al, 2000; See FN16. Davidson et al, 1979.
[69] See FN15. Rabkin et al, 1999; 2000; See FN15. Strawford et al, 1999; See FN15. Sattler et al, 1999; See FN15. Grinspoon et al, 1998; 1999; 2000; Bhasin et al, 2000; See FN15. Van Loan et al, 1999.
[70] Bartsch G, Scheiber K. Tamoxifen Treatment in Oligozoospermia. European Urology. 1981; 7(5): 283-287. and iatrogenic Cushing’s syndrome Cihak RW, Beary FD. Elevated Triiodothyronine and Dextrothyroxine Levels: A Potential Cause of Iatrogenic Hyperthyroidism. Southern Medical Journal. 1977 Feb; 70(2): 256-257. Smidt KP, Johnston E. Undetected Iatrogenic Hypothyroidism: A Late Complication of Radio-Iodine Therapy. New Zealand Medical Journal. 1975 Apr 9; 81: 325-328. Tuel SM, Meythaler JM, Cross LL. Cushing’s Syndrome from Epidural Methylprednisolone. Pain. 1990 Jan; 40(1): 81-84. Kimmerle R, Rolla AR. Iatrogenic Cushing’s Syndrome Due to Dexamethasone Nasal Drops. American Journal of Medicine. 1985 Oct; 79(4): 535-537.
[71] See FN8. Tenover, 1992; See FN4. Bhasin et al, 1996; See FN15. Strawford et al, 1999; See FN28. Marynick et al, 1979.
[72] See FN57. Gazvani et al, 1997; See FN57. Wu et al, 1996.
About the author
The research of Michael Scally focuses on returning individuals to normal physiology after the discontinuation of anabolic steroids. Dr. Scally has presented his medical protocol for the treatment of Anabolic Steroid Induced Hypogonadism before the Endocrine Society, American Association of Clinical Endocrinologists, American College of Sports Medicine, and International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV. Dr. Scally is the author of "Anabolic Steroids - A Question of Muscle: Human Subject Abuses in Anabolic Steroid Research."
Leave a Reply
You must be logged in to post a comment.