Anabolic steroids don’t only affect the endogenous testosterone production, but also sperm production—a process called spermatogenesis. In this article I will walk you through how spermatogenesis works, how anabolic steroid use ties into this, and what you can possibly do about this.
A brief overview of spermatogenesis
Spermatogenesis is tightly regulated by the Leydig and Sertoli cells of the testis. The Leydig cells produce testosterone in response to activation of the LHCG receptor (LHCGR). This receptor is activated by binding of luteinizing hormone (LH). Testosterone, in turn, acts on its neighboring cells, including the Sertoli cells, to control spermatogenesis. Activation of the FSH receptor (FSHR) on the Sertoli cells directly controls spermatogenesis.
The production of sperm takes place in the seminiferous tubules and can be divided in roughly three stages as depicted below:
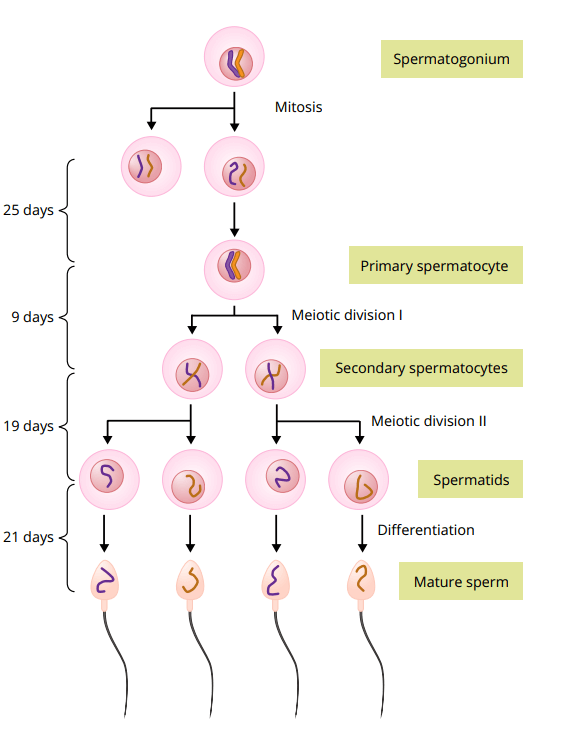
All these stages take place in the seminiferous tubules. During the first stage, the spermatogonia will migrate among Sertoli cells towards the lumen of the tubules. As they migrate along the Sertoli cells, these spermatogonia slowly divide and differentiate into mature sperm cells. First, they undergo mitosis—the division into two identical daughter cells. Some of these daughter cells will undergo further modification and enlargement, a process known as spermatocytogenesis, which will yield the primary spermatocytes. These cells, in turn, will undergo meiosis. Herein, two consecutive cell divisions take place, thus resulting in a total of four daughter cells. Each of these cells will have half the number of chromosomes of the parent cell. After the first cell division we call these cells secondary spermatocytes, and after the second meiotic division we call them spermatids. Finally, the spermatids differentiate into spermatozoa (mature sperm) during spermiogenesis.
The entirety of spermatogenesis takes about 74 days to complete [1]. After that, it will take another 1 to 21 days before the sperm cells end up in the ejaculate [2]. Consequently, when spermatogenesis has stopped, and resumes, it will take a while before this is reflected in a semen analysis.
Spermatogenesis relies heavily on the intratesticular testosterone (ITT) concentration. Given that LH stimulates the testes to produce testosterone, and thus is responsible for the ITT concentration, LH is important for spermatogenesis. Normally, the ITT concentration is roughly 100 higher than in the bloodstream [3]. The administration of 200 mg testosterone enanthate weekly greatly reduces this, to about 2% of baseline levels. While never researched in humans, the lower limit of ITT concentration necessary for a quantitatively normal spermatogenesis in rats is about 20% of baseline [4]. Once it drops below that, there’s a clear relationship between the drop in ITT concentration and sperm count.
Anabolic steroid use suppresses spermatogenesis
Anabolic steroid use suppresses endogenous testosterone production. It does so through negative feedback at the level of the hypothalamus and pituitary. Briefly, the hypothalamus secretes a hormone called gonadotropin-releasing hormone (GnRH) that is released into the hypophyseal portal system. Through this system, it can reach the anterior pituitary. Here, it will bind to its cognate receptor which will lead to secretion of gonadotropins by the anterior pituitary. These gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), hit the systemic circulation which transports them to their target organ: the testicles. The binding of LH to its cognate receptor will lead to testosterone production. Binding of FSH to its cognate receptor plays an important role in spermatogenesis. And, as described above, the produced testosterone is pivotal in spermatogenesis too.
Anabolic steroids inhibit the secretion of GnRH by the hypothalamus and the secretion of gonadotropins by the pituitary. As a result, both testosterone production and sperm production are suppressed. This can lead to a condition named azoospermia, in which no spermatozoa can be found in a semen sample. Or it can lead to oligozoospermia, in which the concentration of spermatozoa is very low (lower than 15 million per mL or 39 million per ejaculate) [5].
For example, in one study, 65% of men became azoospermic within 6 months of testosterone enanthate administration at 200 mg weekly [6]. Since LH and FSH weren’t fully suppressed (-66.7 and -62.5%, respectively), it can be speculated that more men would have become azoospermic with a higher, more suppressive, dosage. Indeed, when combined with a progestin (which would lead to stronger suppression of LH & FSH), azoospermia rates of almost 90% are generally seen [7]. However, a prospective observational trial (the HAARLEM trial) following 100 anabolic steroid users before, during, and at 2 points after their anabolic steroid cycle, saw similar results as the trial in which 65% of men became azoospermic [8]. Data on semen analysis was available for 91 users at the end of their cycle. Despite practically full suppression of LH and FSH in almost all users, sperm concentration was below 15 million per mL in 68% of users (total sperm count was below 40 million in 77%). A key difference here might be the time of suppression, as the other trial showed the cumulative rate of azoospermia up to 6 months, whereas the anabolic steroid users were on for varying amounts of time, with a median duration of 13 weeks. Additionally, some of the anabolic steroid users used hCG during their cycle, which might have stimulated spermatogenesis to some degree (I’ll get back to this later). Although the authors write: “(…) the use of hCG had no detectable effect on testicular size or spermatogenesis”. This might be attributed to underdosed hCG, incorrect usage, or perhaps to some degree or another by lack of statistical power. Finally, high dosages of AAS—contraintuitively—might stimulate some spermatogenesis by replacing some of the missing endogenous androgenic activity that would otherwise take place, as explained in the previous section.
Either way, it’s clear that anabolic steroid use significantly impairs spermatogenesis.
Anabolic steroid use shrinks your balls
The testes comprise the interstitial compartment that houses the Leydig cells, and the seminiferous tubule compartment, that hosts spermatogenesis. The latter is responsible for the bulk of the volume of the testes, with values in the literature varying from 60 up to 90% [9, 10]. A large part of that volume consists of developing sperm cells. Consequently, when spermatogenesis is impaired, the testes will decrease in size. For example, the study I mentioned earlier in which 65% of men became azoospermic within 6 months of testosterone administration saw a decrease in testicular volume of 16.5% [6]. A trial in which testosterone was combined with a very low dosage of an oral progestogen (levonorgestrel) for a stronger suppression saw a larger decrease in testicular volume of about 30% [11]. The HAARLEM trial mentioned earlier saw a 24% decrease. It’s interesting to note that the AAS users saw their testicular volume return to what it was 3 months after cessation of usage (there was only a -4% difference at that point).
Gonadotropin therapy (hCG & hMG/FSH) can preserve spermatogenesis
The effect of hCG and FSH or hMG on spermatogenesis is perhaps most elegantly demonstrated in a series of experiments by Matsumoto et al. [12]. First, healthy male subjects received 5000 IU hCG twice weekly for 7 months. This would strongly stimulate testosterone production on its own and as a consequence FSH was completely suppressed. Regardless, some sperm production was maintained as sperm concentrations were reduced from 88 million/mL to 22 million/mL after 4 months. After these 7 months, testosterone enanthate (200 mg weekly) was added to the hCG for another 6 months in these men. Sperm concentrations remained virtually unaffected (26 million/mL during the last 3 months).
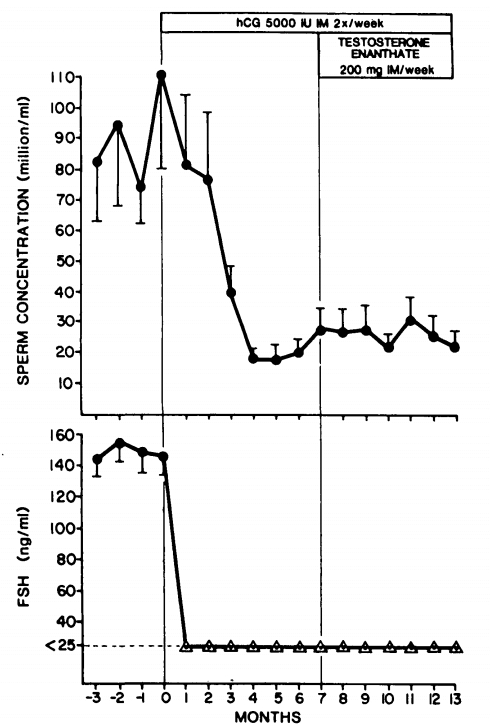
After this period, 4 subjects continued the hCG for another 3 months without testosterone. After this, FSH was added in two of the subjects (100 IU daily), and hMG was added in the other two subjects (75 IU daily). The addition of FSH or hMG led to a strong increase in sperm production, reaching a mean of 103 million/mL during the last 2 months:
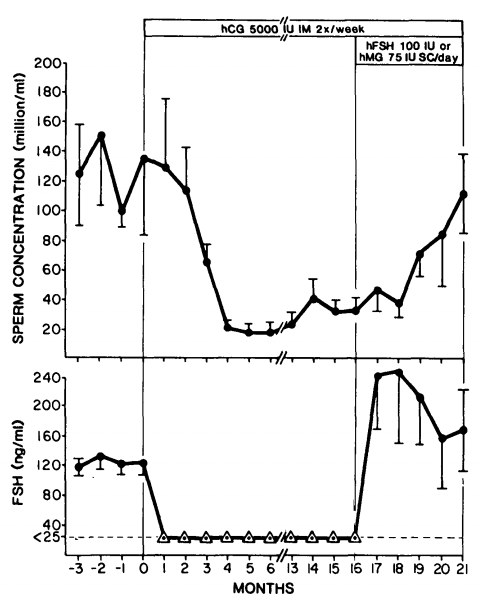
Similarly, FSH alone can preserve some spermatogenesis during suppression by testosterone therapy as illustrated in the picture below [13]:
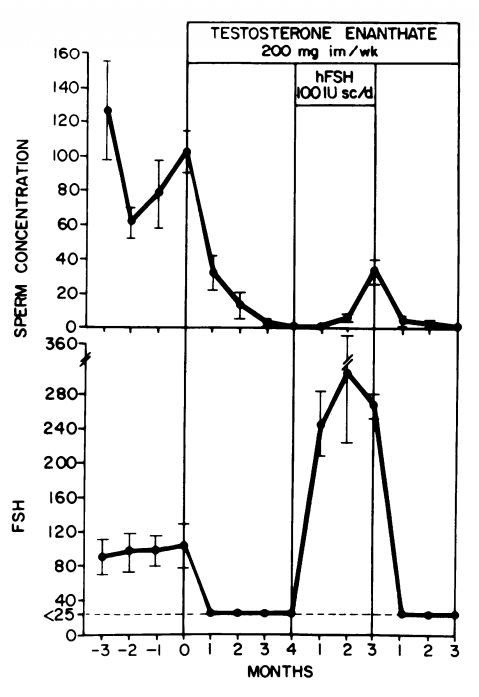
What can be derived from these findings is that both FSH and hCG can preserve some spermatogenesis during gonadotropin suppression by testosterone, but that both are required for a quantitatively normal spermatogenesis. It should be noted, however, that there were marked interindividual differences. In the earlier trial with hCG, one man became azoospermic during hCG treatment.
A small retrospective study suggests that hCG alone, at a dosage of 500 IU every other day, can fully preserve spermatogenesis in conjunction with testosterone replacement therapy [14]. Perhaps there was enough residual FSH secretion in these men to allow for full preservation of spermatogenesis. Additionally, the retrospective nature of the trial might have led to a bias in the results.
This further brings me to another aspect I would like to discuss: dosage. One study found that injecting hCG at dosage of 250 IU every other day resulted in an intratesticular testosterone concentration virtually the same as baseline [15]. Given the pivotal role of intratesticular testosterone in spermatogenesis, one could argue that this low dosage should be sufficient for preservation of spermatogenesis during AAS use. However, this has not been directly researched in a controlled trial.
A higher, but still lower dosage than used in the trials by Matsumoto, demonstrated preservation of some spermatogenesis in patients with secondary hypogonadism with hCG dosed at 500 to 2500 IU twice weekly [16]. The dosages were titrated based on the achieved testosterone levels. Addition of FSH (3x 150 IU hMG weekly) was required for quantitatively normal spermatogenesis. This trial was, again, a retrospective trial though.
Finally, some concerns have been raised about the effect of hCG on sperm morphology. A Finnish study suggests that the concomitant use of hCG and AAS in high dosages can negatively impact sperm morphology [17]. They followed 18 recreational power athletes, of which 16 used hCG in conjunction with high dosages of AAS. Semen samples were taken at the end of the AAS cycle, about 1.5 months post-cycle and about 6 months post-cycle. Of course, sperm production was impaired, with an average sperm count of 33 million/mL at the end of the AAS cycle. One subject became azoospermic (and remained so throughout the subsequent withdrawal period). This indeed seems to demonstrate that hCG usage can preserve some spermatogenesis during AAS use. Sperm morphology, however, was only 15% compared to a 40% average from a Finnish cohort of sperm bank donors. Moreover, they found a correlation between the total dose of hCG used and morphologically abnormal sperm.
When they stratified the users into two groups: a high-dose hCG group (>12.000 IU total) and a low-dose group (<12.000 IU total), they note that there was a significant difference in sperm morphology between the two. On average, 22% was normal in the high-dose group and 72% in the low-dose group right at the end of the AAS cycle. Eh..? If the group average is 15%, how can it be higher in both the high-dose and low-dose group? Something is wrong with the data. So that’s one problem with this study. (Noteworthy; because the high-dose group had an almost five times higher sperm concentration, the absolute amount of morphologically normal sperm was higher in the high-dose group.)
One could argue that it could be the absence of FSH, rather than hCG per se, that could have impacted morphology. Indeed, high hCG doses have been found to improve sperm sperm motility and normal morphology in subfertile men with normal FSH levels [17]. Additionally, one could argue that AAS itself might have a direct negative effect on sperm morphology in high doses [18]. This might not manifest itself if only small amounts of sperm are produced, as was the case in the low-dose group. Matsumoto et al. also demonstrated no effect of hCG (3x 5000 IU weekly) on sperm morphology in conjunction with testosterone in a small study [19]. Finally, abuse of other not-reported substances might have had an impact as well.
Conclusion
Spermatogenesis is under tight regulation of LH & FSH. When AAS are administered, the secretion of these two hormones is greatly suppressed. Consequently, spermatogenesis is greatly suppressed too. In the majority of men this leads to azoospermia. The use of hCG has been found to maintain some spermatogenesis, albeit at a lower level than normal. The addition of FSH (either directly or as a part of hMG) is required to fully preserve spermatogenesis. The dosage required to optimally maintain spermatogenesis on hCG alone during an anabolic steroid cycle is not completely clear. Given the important role of intratesticular testosterone in maintaining spermatogenesis, one might argue that a dosage that sustains this also optimally sustains spermatogenesis. As such, one might arrive at a dosage around 250 IU every other day. However, (controlled) clinical trials directly evaluating the impact on spermatogenesis with suppression of gonadotropins have all used markedly higher dosages. Evidence from retrospective trials suggests that 500 to 2500 IU twice weekly might be sufficient. Ideally, one tests their sperm to see what works for them. Bear in mind that the entire process of spermatogenesis and the subsequent appearance of sperm in the ejaculate can take up to roughly 3 months. Changes in therapy thus might require at least 3 months for their effects to be reflected in sperm analysis.
References
- Amann, Rupert P. “The cycle of the seminiferous epithelium in humans: a need to revisit?.” Journal of andrology 29.5 (2008): 469-487.
- Rowley, Mavis J., Florence Teshima, and Carl G. Heller. “Duration of transit of spermatozoa through the human male ductular system.” Fertility and sterility 21.5 (1970): 390-396.
- McLachlan, Robert I., et al. “Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men.” The Journal of Clinical Endocrinology & Metabolism 87.2 (2002): 546-556.
- Zirkin, Barry R., et al. “Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis.” Endocrinology 124.6 (1989): 3043-3049.
- T. G. Cooper, E. Noonan, S. Von Eckardstein, J. Auger, H. Baker, H. M. Behre, T. B. Haugen, T. Kruger, C. Wang, M. T. Mbizvo, et al. World health organization reference values for human semen characteristics. Human reproduction update, 16(3):231–245, 2010.
- W. H. O. T. F. on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia in normal men. The Lancet, 336(8721):955–959, 1990.
- Page, Stephanie T., John K. Amory, and William J. Bremner. “Advances in male contraception.” Endocrine reviews 29.4 (2008): 465-493.
- Smit, D. L., et al. “Disruption and recovery of testicular function during and after androgen abuse: the HAARLEM study.” Human Reproduction 36.4 (2021): 880-890.
- S. Melmed. Williams textbook of endocrinology. 13th edition. Elsevier Health Sciences, 2016.
- M. Simoni and I. T. Huhtaniemi. Endocrinology of the Testis and Male Reproduction. Springer, 2017.
- Anawalt, Bradley D., et al. “Intramuscular testosterone enanthate plus very low dosage oral levonorgestrel suppresses spermatogenesis without causing weight gain in normal young men: a randomized clinical trial.” Journal of andrology 26.3 (2005): 405-413.
- Matsumoto, Alvin M., Anthony E. Karpas, and William J. Bremner. “Chronic human chorionic gonadotropin administration in normal men: evidence that follicle-stimulating hormone is necessary for the maintenance of quantitatively normal spermatogenesis in man.” The Journal of Clinical Endocrinology & Metabolism 62.6 (1986): 1184-1192.
- Matsumoto, Alvin M., et al. “Reinitiation of sperm production in gonadotropin-suppressed normal men by administration of follicle-stimulating hormone.” The Journal of clinical investigation 72.3 (1983): 1005-1015.
- Hsieh, Tung-Chin, et al. “Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy.” The Journal of urology 189.2 (2013): 647-650.
- Coviello, Andrea D., et al. “Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression.” The Journal of Clinical Endocrinology & Metabolism 90.5 (2005): 2595-2602.
- Depenbusch, Marion, et al. “Maintenance of spermatogenesis in hypogonadotropic hypogonadal men with human chorionic gonadotropin alone.” European journal of endocrinology 147.5 (2002): 617-624.
- Homonnai, Z. T., M. Peled, and G. F. Paz. “Changes in semen quality and fertility in response to endocrine treatment of subfertile men.” Gynecologic and obstetric investigation 9.5 (1978): 244-255.
- Torres-Calleja, J., et al. “Effect of androgenic anabolic steroids on sperm quality and serum hormone levels in adult male bodybuilders.” Life sciences 68.15 (2001): 1769-1774.
- Matsumoto, Alvin M., et al. “Human chorionic gonadotropin and testicular function: stimulation of testosterone, testosterone precursors, and sperm production despite high estradiol levels.” The Journal of Clinical Endocrinology & Metabolism 56.4 (1983): 720-728.
Main photo de Nadezhda Moryak provenant de Pexels
About the author
Peter Bond is a scientific author with publications on anabolic steroids, the regulation of an important molecular pathway of muscle growth (mTORC1), and the dietary supplement phosphatidic acid. He is the author of several books in Dutch and English, including Book on Steroids and Bond's Dietary Supplements.

Leave a Reply
You must be logged in to post a comment.