Perhaps one of the most worrying side effects of prolonged anabolic steroid use are those related to the heart. Indeed, a startling amount of case reports associating anabolic steroid use and adverse heart events have been published in the literature [1]. Most frequently, this included myocardial infarction. While case reports obviously can’t establish anabolic steroid use as a causal factor, there is plenty of other evidence in the literature which, at the least, should raise flags of concern. In particular, a large body of evidence relates anabolic steroid use in high dosages to structural and functional changes of the heart. By extension, these cardiac changes might be related to what’s observed clinically in the aforementioned case reports.
In this article I will narrate out how anabolic steroid use in high dosages appears to alter the structure and function of the heart and how this might relate to disease. But before I do this, I’ll provide you with a little primer on how the heart functions. After all, this is essential in understanding these adverse cardiac changes that might be caused by anabolic steroid use.
The heart cycle
Throughout life your heart ensures that all your cells are supplied with adequate nutrients and oxygen. It does so by continuously pumping blood through your cardiovascular system. The heart itself, in essence, is split up into two separate pumps. The left and right side of the heart each serve its own purpose as a pump. They’re separated by a ‘wall’ called the interventricular septum. The right side pumps deoxygenated blood through the lungs in order to reoxygenate it. The left side pumps the oxygenated blood through the systemic circulation, thereby providing blood flow to all cells of the body.
In turn, the left and the right side of the heart are each composed of two chambers. A chamber on top, called the atrium, and a chamber on the bottom, called the ventricle. The atrium assists its ventricle by making sure enough blood enters it. The ventricle, in turn, is responsible for pumping out this blood through either the pulmonary circulation (right ventricle) or the systemic circulation (left ventricle).
Let’s have a look at the picture below which illustrates this and then I’ll take you through how this blood flows through the heart with each and every heartbeat. If you already know how the heart cycle is established, you can skip this entire section and move on to the next.
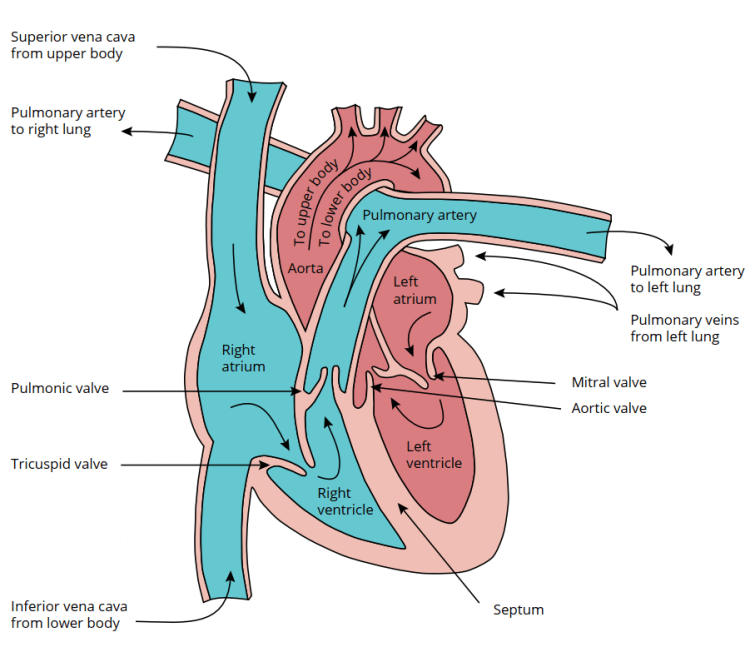
The right atrium receives deoxygenated blood from both the lower and upper body. The blood from the upper body, such as from the brain, enters it from the top side through the superior vena cava. Similarly, blood that has just traveled through your legs and other parts of your lower body come together in the inferior vena cava and drain into the right atrium. The right atrium thus collects blood that has just passed through all your organs and tissues and from which some nutrients and oxygen have been taken up from.
Separating the right atrium and the right ventricle is a valve. This valve, called the right atrioventricular (AV) valve or tricuspid valve, opens up when the pressure in the right ventricle is lower than the pressure in the right atrium. This happens shortly after the right ventricle has finished contracting, i.e. at the end of systole. Consequently, the phase of diastole begins. Once the right AV valve opens, blood flows continually from the superior vena cava and inferior vena cava straight through the right atrium into the right ventricle. Finally, the right atrium also contracts, squeezing some additional blood into the right ventricle before the right ventricle starts contracting again and closes the AV valve. Once this happens, the systolic phase starts again and the contraction of the right ventricle builds up enough pressure to open the valve that separates the right ventricle from the pulmonary circulation. This valve, called the pulmonic valve, then opens and blood flows through the pulmonary artery that branches into the right and left lung. The deoxygenated blood will then exchange oxygen and carbon dioxide with the lungs. Once it has traveled through the lungs, it will enter the left atrium. Here, the same thing basically happens as with the right side of the heart. Once the left ventricle is done contracting, the left AV valve opens, and blood flows through the left atrium into the left ventricle. Then, the left atrium squeezes some additional blood into the ventricle by contracting. Finally, the left ventricle starts contracting, the left AV valve (also called the mitral valve) closes, and pressure builds up until the valve separating the left ventricle from the systemic circulation (the aortic valve) opens. The oxygenated blood then flows through your entire body and ultimately enters the heart again in the right atrium.
The function of the heart can be split into two things: the diastolic function (the filling of the heart with blood) and the systolic function (the pumping of the blood through the pulmonary circulation and the systemic circulation). Anabolic steroids can affect this functioning of the heart. Additionally, anabolic steroids affect the structure of the heart, such as the thickness of the walls that make up the chambers. Such changes have also been associated with mortality or adverse cardiac events.
The application of echocardiography in anabolic steroid users
Echocardiography can be used to paint a picture of the structure and function of the heart. The technique uses ultrasound to examine this. It’s the same technique that is used to show the baby in the belly of a pregnant woman. Researchers started to apply this technique to anabolic steroid users in the 1980’s.
For historical purposes, I’ll discuss the first trial which applied echocardiography to bodybuilders using anabolic steroids. The study also makes a good case to highlight some of the caveats when interpreting research like this. The first trial was described in a paper published by Salke et al. in 1985 [2]. Their publication was titled “Left ventricular size and function in body builders using anabolic steroids”. They applied echocardiographic measurements to three groups:
- AAS-using bodybuilders
- non-AAS-using bodybuilders
- an inactive control group
They made measurements of the left ventricle, such as left ventricular septal and posterior wall thicknesses. They then also calculated the ratio between these measurements. When these walls are too thick, it can be a sign of cardiomyopathy and a disproportionately large septal thickness compared to posterior wall thickness is common in many diseases [3]. Additionally, they measured its internal dimensions both at the end of systole and diastole. This allowed them to also calculate, for example, the shortening fraction (or fractional shortening). This is a functional index: basically the percentage with which the ventricle decreases in size during systole. If the fractional shortening decreases, it’s a sign of systolic dysfunction. After all, it implies the ventricle is less efficient at pumping blood.
Either way, I’m drifting off a bit. The study found no significant differences in any of these measurements between the AAS-using bodybuilders and non-AAS-using bodybuilders. But there are plenty of caveats. First, the group sizes were small (15 subjects per group). This makes it harder to find a statistically significant difference, even if there is a true difference. As such, true differences might simply not have reached statistical significance. Having said that, the left ventricular septal thickness was 13.7, 12.4, and 9.2 mm in the AAS-using bodybuilders, non-AAS-using bodybuilders, and inactive control group, respectively. This is quite thick. The normal reference range for left ventricular septal thickness is 6 to 10 mm [4]. However, athletes have thicker LV walls than nonathletes. As such, alternative cutoffs have been proposed for athletes, with a cutoff of 12 mm for Caucasian athletes, and a cutoff of 14 mm for black-African or Afro-Caribbean athletes [5]. Even with these cutoffs for athletes it’s obvious that bodybuilders had a pretty damn thick LV septum, especially the AAS-using one. Considering these were the average values, it was even thicker in some.
The left ventricular posterior wall thickness was identical for both bodybuilder groups at 9.4 mm. (Normal range is also 6-10 mm [4].) However, given the thicker septum in the AAS-using ones, the ratio between the septal thickness and posterior wall thickness was higher in them. Which could be a sign of cardiomyopathy. Either way, despite the lack of statistical significance, these numbers aren’t comforting.
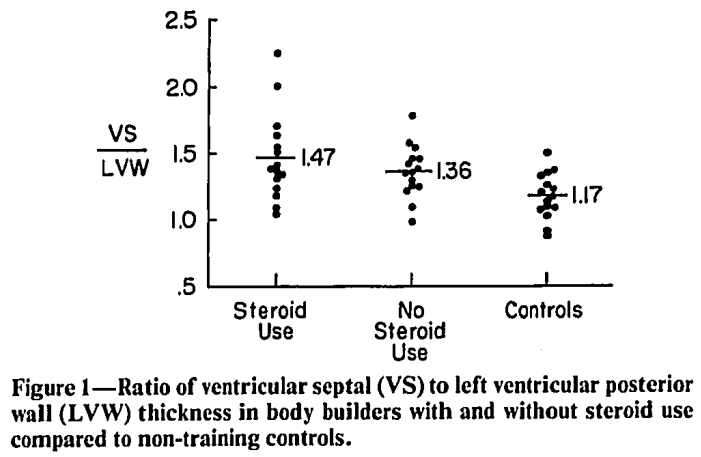
Something that might have affected the measurements is the fact that the study wasn’t blinded. Meaning, the ones that were performing the echocardiography were aware whether or not the subject they were examining was using steroids. This opens the door to something called observer bias. Moreover, the study was cross-sectional in nature. As such, the measurements were taken at one particular moment in time, and it’s hard to derive any causality from it—contrary to prospective, especially interventional, trials. There are even more caveats to this study, such as the technique used to assess the wall thicknesses, but that will make this article more lengthy and technical than it needs to be. The message that I’m trying to get across is that there are a lot of variables to take into account when interpreting a paper like this.
So what does the rest of the literature tell us? It’s gonna be an extremely long and boring article if I’ll be discussing each publication separately to the extent that I just did with the echocardiographic findings of Salke et al. As such, I’m gonna give you the distilled summary of my interpretation of the literature. The aggregate of the literature suggests that long-term AAS usage in high dosages might cause an increase in left ventricular thickness, left ventricular mass (even when corrected for body surface area), suggesting a slight to moderate left ventricular concentric hypertrophy. Similarly, measurements of heart function suggest an impairment of diastolic and systolic function. Some evidence seems to suggest that the degree to which these changes occur correlate with AAS dosage and duration. There’s no clear evidence to indicate that one AAS is more detrimental than another in this regard. More research is required for that. Finally, these changes seem to be, at least partially, reversible when AAS usage is stopped. I’ve summarized all echocardiographic studies I could find up to 2019 in a table for my book, Book on Steroids. I’ve copied it here so you can glance over it. (The book also goes into depth in most of these studies.)
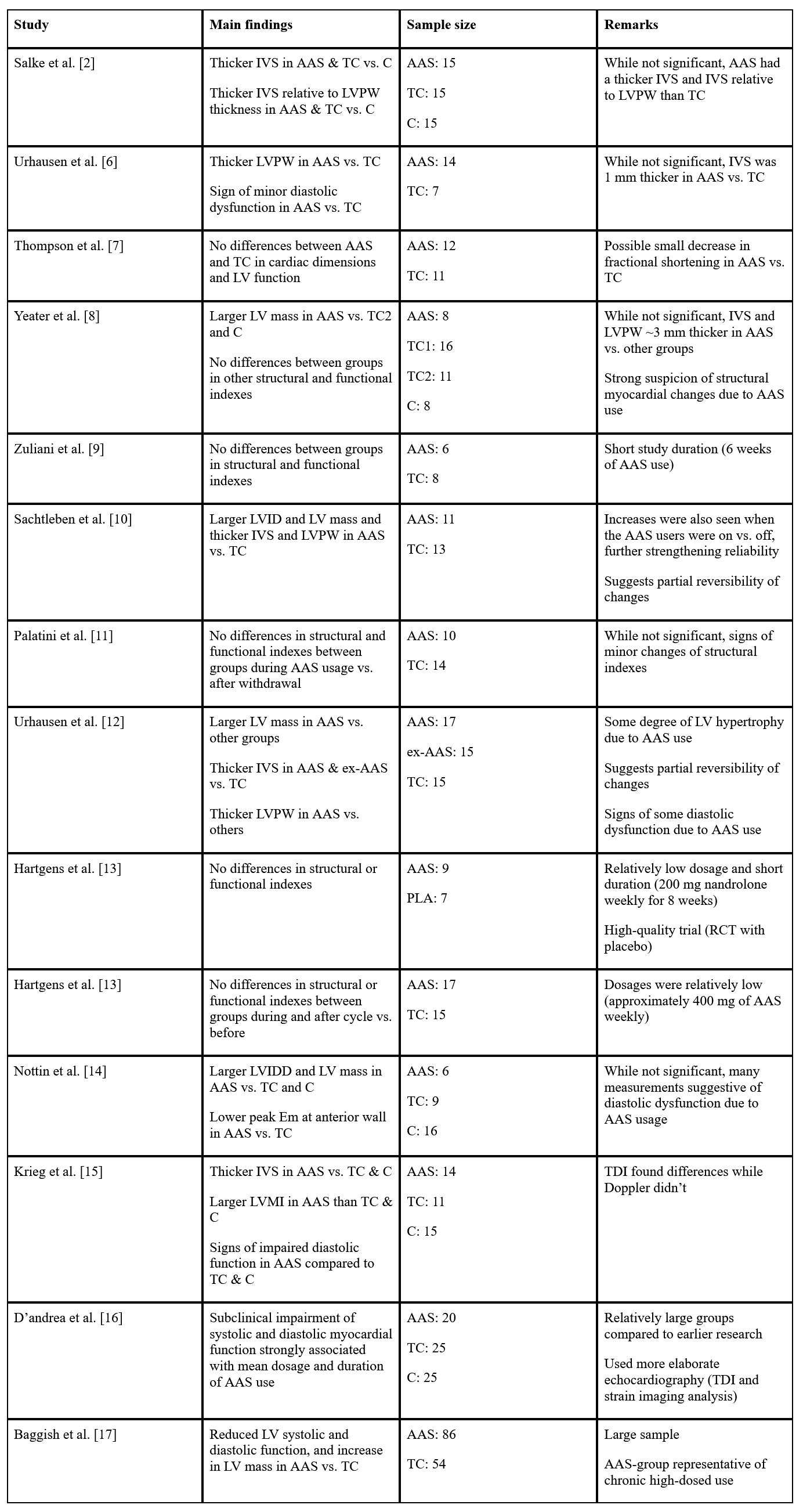
Abbreviations: TC, trained control group; C, untrained or non-resistance-trained control group; PLA, placebo group; IVS, interventricular septum; LVPW, left ventricular posterior wall; LV, left ventricle; LVID, left ventricular internal diameter; LVIDD, left ventricular internal diastolic diameter; LVMI, left ventricular mass index; TDI, Tissue Doppler Imaging; RCT, randomized controlled trial.
Results from the HAARLEM trial
The HAARLEM trial was a prospective study conducted by the outpatient clinic for anabolic steroid users in the Netherlands [18]. In brief, 100 anabolic steroid users were followed over time while they self-administered AAS. Mean dosage, based on label information, was 898 mg per week, thus making their AAS cycle quite representative of common usage by bodybuilders. Measurements were taken before, during, as well as 3 months after the end of their cycle and 1 year after the start of their cycle. A total of 31 subjects of this sample also underwent echocardiographic measurements. Notably, they applied 3D echocardiography with some badass equipment (Philips Epiq 7). Due to the prospective nature of this trial, the good sample size, the modern equipment, and the representative AAS usage of the subjects, this trial delivers some high-quality data.
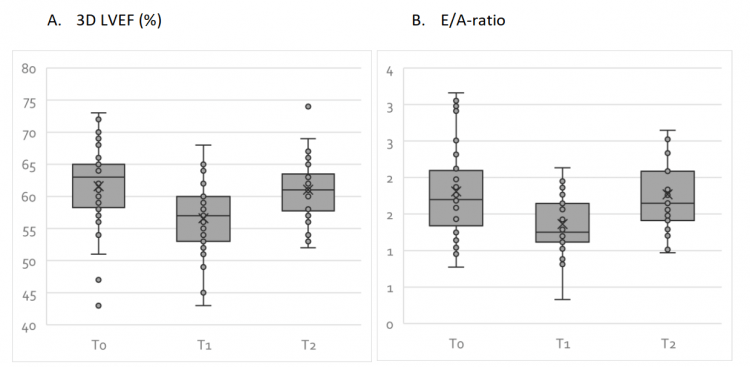
While I’m writing this, the echocardiographic findings of the HAARLEM trial haven’t been published yet. [All of the following findings are through personal communication with DL Smit.] I’ll go over the main findings. What they found was that 3D left ventricular ejection fraction declined 4.9 %. The ejection fraction is the percentage of blood that’s squeezed out of the ventricle during systole. A normal ejection fraction lies between 52 and 72 % [19]. A decrease simply means the left ventricle is pumping its blood less well. Almost all subjects still had their ejection fraction within normal range, so they’re unlikely to have noticed anything from it.
The E/A-ratio declined with 0.45. So what’s the E/A ratio? Remember in the section on the heart cycle how I discussed how blood rapidly flows into the left ventricle once the left AV valve opens? The peak blood velocity at which this happens is a parameter called the Emax. I also mentioned how the atrium squeezes after this, to get a little bit of additional blood in the ventricle before the valve closes again. The peak blood velocity at which this happens is called the Amax value. And now you’ve guessed it, the ratio between the Emax and Amax value is called the E/A ratio. A decreased E/A ratio is indicative of diastolic dysfunction, i.e. the left ventricle has a harder time getting filled with blood which could mean it has become more stiff.
The 3D left atrial volume increased with 9.2 mL. An increase in left atrial volume can be indicative of left ventricular diastolic function [20]. Left ventricular mass increased with 28.3 g and the increase could be attributed to an increase in interventricular septum as well as posterior wall thickness. The increase was found to be positively correlated with AAS average weekly dose. Notably, after a median recovery time of 8 months, all parameters returned to baseline.
Conclusion
Prolonged anabolic steroid use can affect the structure and function of the heart. These changes appear, at least partly, reversible after cessation of use. It’s hard to translate these findings to concrete numbers which express the risk of heart issues or even mortality with it. Nevertheless, it’s clear that these changes are detrimental to an AAS user’s health. As such, it seems advisable to take an annual echocardiographic assessment to monitor potentially unfavorable changes to cardiac structure and function.
References
- Vanberg, Paul, and Dan Atar. “Androgenic anabolic steroid abuse and the cardiovascular system.” Doping in Sports: Biochemical Principles, Effects and Analysis (2010): 411-457.
- Salke, Richard C., Thomas W. Rowland, and Edmund J. Burke. “Left ventricular size and function in body builders using anabolic steroids.” Medicine and science in sports and exercise 17.6 (1985): 701-704.
- Kansal, S., D. Roitman, and L. T. Sheffield. “Interventricular septal thickness and left ventricular hypertrophy. An echocardiographic study.” Circulation 60.5 (1979): 1058-1065.
- Lang, Roberto M., et al. “Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging.” European Heart Journal-Cardiovascular Imaging 16.3 (2015): 233-271.
- Brosnan, Maria J., and Dhrubo Rakhit. “Differentiating athlete’s heart from cardiomyopathies—the left side.” Heart, Lung and Circulation 27.9 (2018): 1052-1062.
- Urhausen, A., R. Hölpes, and W. Kindermann. “One-and two-dimensional echocardiography in bodybuilders using anabolic steroids.” European journal of applied physiology and occupational physiology 58.6 (1989): 633-640.
- Thompson, Paul D., et al. “Left ventricular function is not impaired in weight-lifters who use anabolic steroids.” (1992): 278-282.
- Yeater, Rachel, et al. “Resistance trained athletes using or not using anabolic steroids compared to runners: effects on cardiorespiratory variables, body composition, and plasma lipids.” British journal of sports medicine 30.1 (1996): 11-14.
- Zuliani, U., et al. “Effects of Anabolic Steroids, Testosterone, and HGH on Blood Lipids and Echocardiography Parameters in Body Builders.” International journal of sports medicine 10.01 (1989): 62-66.
- Sachtleben, Thomas R., et al. “The effects of anabolic steroids on myocardial structure and cardiovascular fitness.” Medicine and science in sports and exercise 25.11 (1993): 1240-1245.
- Palatini, Paolo, et al. “Cardiovascular effects of anabolic steroids in weight‐trained subjects.” The Journal of Clinical Pharmacology 36.12 (1996): 1132-1140.
- Urhausen, Axel, T. Albers, and W. Kindermann. “Are the cardiac effects of anabolic steroid abuse in strength athletes reversible?.” Heart 90.5 (2004): 496-501.
- Hartgens, F., E. C. Cheriex, and H. Kuipers. “Prospective echocardiographic assessment of androgenic-anabolic steroids effects on cardiac structure and function in strength athletes.” International journal of sports medicine 24.05 (2003): 344-351.
- Nottin, Stéphane, et al. “Cardiovascular effects of androgenic anabolic steroids in male bodybuilders determined by tissue Doppler imaging.” The American journal of cardiology 97.6 (2006): 912-915.
- Krieg, A., et al. “Cardiac tissue Doppler in steroid users.” International journal of sports medicine 28.08 (2007): 638-643.
- D’Andrea, Antonello, et al. “Left ventricular early myocardial dysfunction after chronic misuse of anabolic androgenic steroids: a Doppler myocardial and strain imaging analysis.” British journal of sports medicine 41.3 (2007): 149-155.
- Baggish, Aaron L., et al. “Cardiovascular toxicity of illicit anabolic-androgenic steroid use.” Circulation 135.21 (2017): 1991-2002.
- Smit, Diederik L., et al. “Baseline characteristics of the HAARLEM study: 100 male amateur athletes using anabolic androgenic steroids.” Scandinavian journal of medicine & science in sports 30.3 (2020): 531-539.
- Nagueh, Sherif F., et al. “Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging.” European Journal of Echocardiography 17.12 (2016): 1321-1360.
- Simek, Christopher L., et al. “Relationship between left ventricular wall thickness and left atrial size: comparison with other measures of diastolic function.” Journal of the American Society of Echocardiography 8.1 (1995): 37-47.
Photo by Tanya Pro on Unsplash
About the author
Peter Bond is a scientific author with publications on anabolic steroids, the regulation of an important molecular pathway of muscle growth (mTORC1), and the dietary supplement phosphatidic acid. He is the author of several books in Dutch and English, including Book on Steroids and Bond's Dietary Supplements.

Leave a Reply
You must be logged in to post a comment.