Muscle memory refers to growing muscle more efficiently/faster after a period of detraining/atrophy than before. So if you’ve had bigger muscles in the past, it will help you to grow them big again at a later point in time. The concept of muscle memory leans for a good part on something called myonuclear permanence. The ‘myo’ in ‘myonuclear’ refers to ‘muscle’ and the ‘nuclear’ doesn’t refer to nuclear reactors, but to the word ‘nucleus’: an organelle of the cell. Before we further explore the concept of muscle memory, and how anabolic steroids tie into this, let’s first cover some background on muscle nuclei or myonuclei.
Some background information on muscle nuclei/myonuclei
Muscles consist of a whole bunch of muscle fibers. Each muscle fiber, or muscle cell, contains multiple nuclei—the organelle of a cell that contains DNA and is the location where the process of gene transcription takes place. Most other human cell types contain only one nucleus, or in some cases even no nucleus at all (red blood cells). To give you an idea about how many nuclei we’re talking about: rat muscle fibers contain about 44 to 116 nuclei per millimeter fiber length, with type 1 muscle fibers containing more nuclei per millimeter than type 2 muscle fibers [1]. The number appears lower in humans, with one researcher reporting about 30 nuclei per millimeter fiber length in brachial biceps muscle [2]. As such, muscle fibers can contain up to thousands of myonuclei as they can span several centimeters in length.
Because the cell nuclei of muscle fibers are unable to divide (i.e. they’re terminally differentiated), muscle fibers rely on surrounding satellite cells for the addition of new nuclei. Essentially, satellite cells are muscle fiber stem cells that can be found squeezed between the sarcolemma (the cell membrane of a muscle fiber) and the basal lamina (a layer of extracellular matrix that’s wrapped around the sarcolemma). They were first discovered and described by Alexander Mauro in scientific literature in 1961 [3]. Using an electron microscope, he saw cells ‘wedged’ between the sarcolemma of frog muscle fibers and the basal lamina. He described them to have a paucity of cytoplasm, with the nucleus making up almost the entire satellite cell volume. He continued to speculate about the origin and the role of satellite cells, briefly touching upon the idea that they might be involved in responding to trauma inflicted on a muscle fiber. Which, indeed, they are [4].
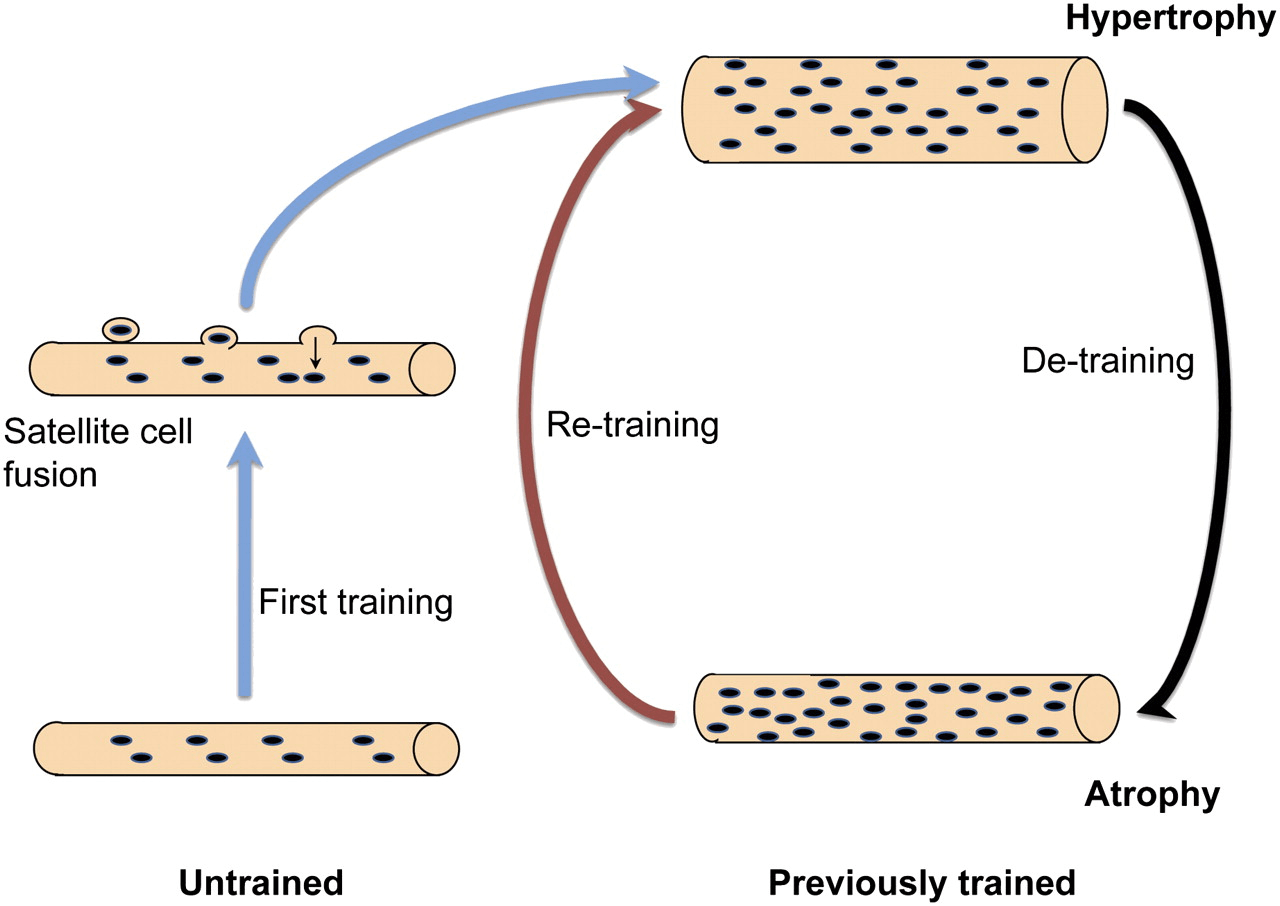
The myonuclear domain hypothesis and myonuclear permanence
The discovery of satellite cells and their role in muscle regeneration beg the question to what extent satellite cells are involved in hypertrophy. A hypothesis named the myonuclear domain hypothesis hooked into this. It posits that a myonucleus controls a limited amount of cytoplasm, and thus for muscle growth to take place, myonuclei need to be added to the muscle fiber to support this. Three key observations supported this hypothesis, namely:
-
γ-radiation exposure makes satellite cells unable to divide and strongly inhibits overload hypertrophy in animal models, while keeping cell metabolism or protein synthesis intact [5].
-
Products (organelle, membrane and structural proteins) derived from a nucleus remain localized to its vicinity [6].
-
The cytoplasm/myonucleus ratio remains fairly constant [7].
This would imply that myonuclear number would increase with growth of a muscle fiber (hypertrophy), while it would decrease with a loss of size of a muscle fiber (atrophy). However, various animal studies suggest that myonuclei aren’t lost during atrophy [8]. As such arose the paradigm of myonuclear permanence: once myonuclei are gained with hypertrophy, they aren’t lost again with detraining. This could potentially allow muscle fibers to regrow more efficiently during subsequent retraining and thus serve as a mechanism of ‘muscle memory’.
Anabolic steroids and myonuclear permanence
So what about anabolic steroids? It’s clear that anabolic steroid use increases myonuclei number. Increasing dosages of testosterone enanthate lead to an increase in myonuclei number per mm of muscle fiber [10]. This is not too surprising: you simply see this with practically all modes of hypertrophy. (And more testosterone is more muscle hypertrophy.)

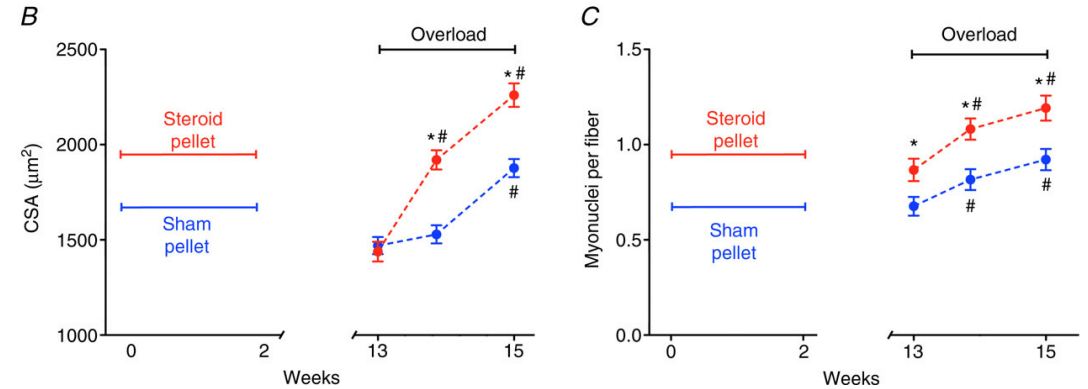
But what about permanence? Do these myonuclei stick around once muscle mass decreases again? In an animal experiment, female mice were exposed to testosterone propionate for 2 weeks, which led to a 66% increase in the number of myonuclei and a 77% increase in the muscle fiber CSA [11]. Muscle mass returned to normal after subsequent withdrawal of the testosterone, but the number of myonuclei remained increased for at least 3 months. 3 months might not sound like a lot, but on the timescale of a mouse it is: the mice they used live for about 2 years. Anyhow, after these 3 months, when the mice were subjected to overload hypertrophy, muscle fiber CSA increased by 30% after 6 days, whereas that of the control mice didn’t increase significantly. After this, muscle mass increased in parallel between both groups, but the CSA was stil 20% higher in the group that was previously exposed to testosterone after 14 days. While this doesn’t prove a causal link between the higher myonuclei number and hypertrophy, it is nevertheless an interesting observation.
What about humans? Two studies have evaluated this and these have been brought to my attention by Alexander Kolliari-Turner, a PhD student at the School of Sport and Health Sciences of the University of Brighton in the United Kingdom. One is a master’s thesis and the other is a PhD thesis.
In the PhD thesis by Anders Eriksson [12], four groups of subjects were recruited. A group of sedentary subjects that served as a control (group C), a group of natural powerlifters (group P), a group of powerlifters using anabolic steroids (group PAS), and a group of powerlifters who previously have used anabolic steroids (group PREV). Myonuclei per muscle fiber were determined in the vastus lateralis and trapezius muscles. The PREV group had discontinued their anabolic steroid use for at least a year (with a mean of 8 years). Indeed, the muscle fiber area measured in the PREV group was comparable to that in the P group, and notably smaller than in the PAS group.
The nuclear domain size (nr. of nuclei per fiber divided by fiber area) distribution per group can be found in the image below. If there was myonuclei permanence, you’d expect a smaller nuclear domain, i.e. more nuclei relative to fiber area, in the PREV group compared to the other groups.
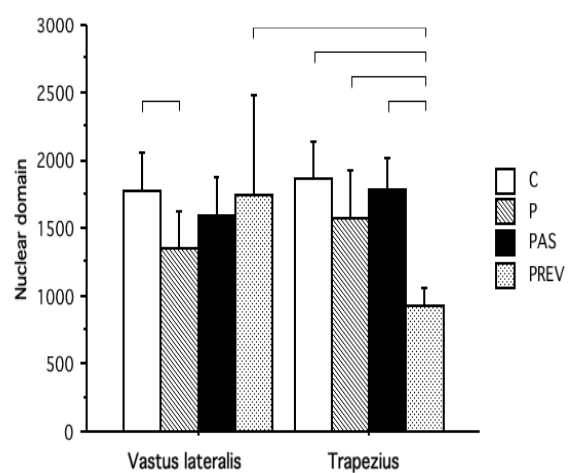
Clearly this is not the case in the vastus lateralis, but it is the case in the trapezius. It’s hard to say what causes this apparent discrepancy between both muscles. Either some property that differs between the two muscles, or its way of use since cessation of AAS use, possibly led to apparent myonuclear permanence in the trapezius muscle.
It should be noted, however, that this was a cross-sectional study with a small number of subjects (32 in total). Ideally you’d have a prospective study evaluating this, although that’s exceedingly hard over long periods of time, as it might take at least a year or longer before changes become apparent. Alternatively, a cross-sectional study with a larger subject pool would be pretty appealing too. Regardless, this does lend some credibility to myonuclei permanence in humans as a result of anabolic steroid use in selected muscles.
In a master’s thesis by Lindholm et al., three groups of subjects were recruited: current anabolic steroid users (group CAS), former anabolic steroid users (group FAS), and resistance-trained controls (group CON) [13]. The former anabolic steroid users had quit their anabolic steroid use for a mean of 6.5 years. In this study, only biopsies of the vastus lateralis muscle were taken. Notably, there were no significant differences in muscle fiber CSA between the three groups. This is undoubtedly the result of the relatively small group sizes (34 subjects in total; a type 2 error).
A small, but significant, difference in myonuclear domain was found between the type 2 muscle fibers of the FAS group compared to the CON group, as can be seen in the figure below:
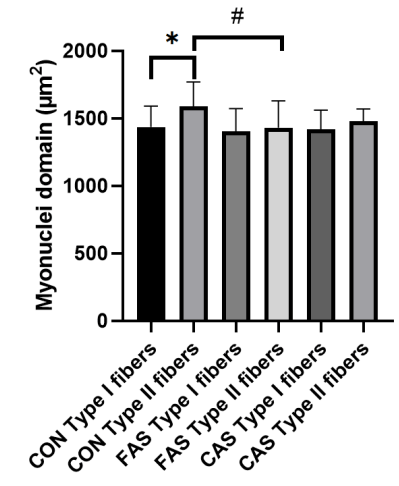
Does this suggest myonuclear permanence? Perhaps. The difference was small and can easily be accounted for by the cross-sectional nature of the study too (and there was no difference compared to the current anabolic steroid users).
Evidence so far is scarce. Either way, when looking at myonuclear permanence in general, overall evidence indicates this to hold in short-term, but evidence is lacking for long-term [14]. Moreover, it’s unclear whether or not myonuclear permanence might aid in subsequent retraining. And given the above data, the debate on whether or not anabolic steroid use leads to muscle memory as a result of myonuclear permanence, is far from settled.
As a concluding remark: there’s also a concept of muscle memory based on something else than myonuclear permanence, namely, epigenetic memory [15]. Briefly, this refers to changes made to the DNA without affecting its nucleotide sequence—so without changing the genetic code. This involves the addition (or removal) of methyl groups to cytosine and adenine nucleotides or histone modifications (e.g. methylation or acetylation of amino acid residues of the histone proteins). The result of this is that it affects gene expression. This would perhaps be something for a future article, as more research is gradually being published about this new exciting avenue.
References
-
Tseng, Brian S., Christine E. Kasper, and V. Reggie Edgerton. “Cytoplasm-to-myonucleus ratios and succinate dehydrogenase activities in adult rat slow and fast muscle fibers.” Cell and tissue research 275.1 (1994): 39-49.
-
Schmalbruch H. Skeletal Muscle. Berlin: Springer-Verlag; 1985.
-
Mauro, Alexander. “Satellite cell of skeletal muscle fibers.” The Journal of Cell Biology 9.2 (1961): 493-495.
-
Forcina, Laura, et al. “An overview about the biology of skeletal muscle satellite cells.” Current genomics 20.1 (2019): 24-37.
-
Rosenblatt, J. David, David Yong, and David J. Parry. “Satellite cell activity is required for hypertrophy of overloaded adult rat muscle.” Muscle & nerve 17.6 (1994): 608-613.
-
Pavlath, Grace K., et al. “Localization of muscle gene products in nuclear domains.” Nature 337.6207 (1989): 570-573.
-
Allen, David L., Roland R. Roy, and V. Reggie Edgerton. “Myonuclear domains in muscle adaptation and disease.” Muscle & nerve 22.10 (1999): 1350-1360.
-
Gundersen, Kristian, and Jo C. Bruusgaard. “Nuclear domains during muscle atrophy: nuclei lost or paradigm lost?.” The Journal of physiology 586.11 (2008): 2675-2681.
-
Bruusgaard, Jo C., et al. “Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining.” Proceedings of the National Academy of Sciences 107.34 (2010): 15111-15116.
-
Sinha-Hikim, Indrani, et al. “Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men.” American Journal of Physiology-Endocrinology and Metabolism 285.1 (2003): E197-E205.
-
Egner, Ingrid M., et al. “A cellular memory mechanism aids overload hypertrophy in muscle long after an episodic exposure to anabolic steroids.” The Journal of physiology 591.24 (2013): 6221-6230.
-
Eriksson, Anders. Strength training and anabolic steroids: a comparative study of the trapezius, a shoulder muscle and the vastus lateralis, a thigh muscle, of strength trained athletes. PhD Diss. 2006.
-
Lindholm, Jesper Bøgh, et al. Effects of Long-Term Supplementation of Androgen Anabolic Steroids on Human Skeletal Muscle – Evidence for Muscle Memory? Master’s Thesis, 2019.
-
Snijders, Tim, et al. “The concept of skeletal muscle memory: Evidence from animal and human studies.” Acta Physiologica 229.3 (2020): e13465.
-
Seaborne, Robert A., et al. “Human skeletal muscle possesses an epigenetic memory of hypertrophy.” Scientific reports 8.1 (2018): 1-17.
About the author
Peter Bond is a scientific author with publications on anabolic steroids, the regulation of an important molecular pathway of muscle growth (mTORC1), and the dietary supplement phosphatidic acid. He is the author of several books in Dutch and English, including Book on Steroids and Bond's Dietary Supplements.

Leave a Reply
You must be logged in to post a comment.