
Having a high blood pressure, also known as hypertension, is one of the most frequent (contributing) causes of death and cardiovascular complications in the world. When measured, it’s split up into systolic pressure (the top number) and diastolic pressure (the bottom number). The systolic blood pressure is the highest pressure reached during contraction of the heart, whereas the diastolic pressure is the lowest pressure reached during relaxation of the heart. A typical blood pressure reading could be 120/80 mmHg, meaning, a systolic pressure of 120 mmHg and a diastolic pressure of 80 mmHg. (The unit, millimeters of mercury [Hg] dates back to when blood pressure was still measured using mercury-containing manometers.)
In order to quantify how bad hypertension is, let’s have a look at a landmark paper published in the Lancet in 2002 that has been cited over a whopping 12.000 times according to Google Scholar [1]. In this paper, the researchers pooled together the individual patient data from 61 prospective observational studies. This included about one million adults with no previous vascular disease at baseline. As such, they had really amazing data to work with and draw conclusions from.
So what did the data show? It showed that mortality from coronary heart disease and stroke increases with a systolic pressure higher than 115 mmHg and a diastolic pressure higher than 75 mmHg. Every 20 mmHg increase in systolic blood pressure and every 10 mmHg increase in diastolic blood pressure beyond these values, doubles mortality due to coronary heart disease and stroke. Meaning, someone with a systolic blood pressure of 135 mmHg has twice the risk of dying from coronary heart disease or stroke compared to someone with a systolic blood pressure of 115 mmHg. That’s a notable difference. This relationship between blood pressure and mortality due to, for example stroke, is pictured in the image below:
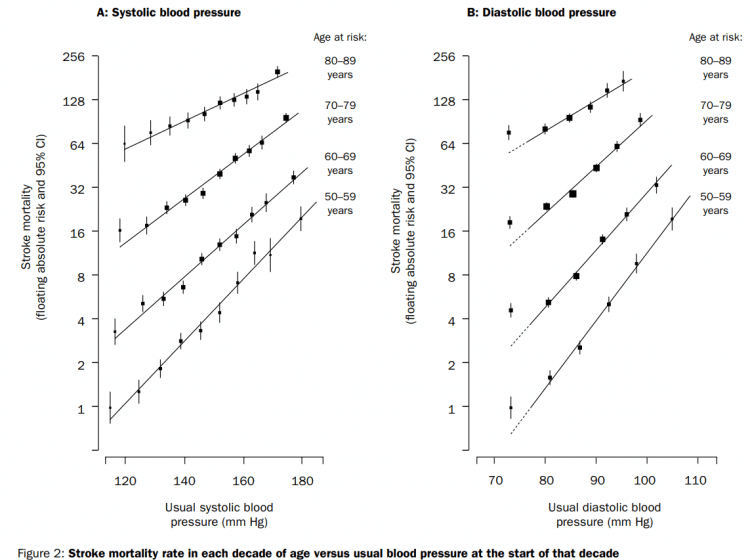
Note that the likelihood of dying from stroke increases steeply with increasing age as well. Which makes sense, of course. While not many people die from stroke in their 40’s, it’s a lot more common in the elderly. As such, the relative risk increase from hypertension becomes more relevant with increasing age as the absolute risk is a lot higher.
In addition to this clear increase in mortality as a result of cardiovascular events, hypertension leads to structural and functional alterations of several organs, and thereby damages them. This target organ damage includes, besides the heart and vasculature, the brain, eyes and kidneys. The target organ damage can, besides the fatal and nonfatal cardiovascular events, manifest itself in retinopathy, dementia, ischemia, albuminuria, glomerulopathy, and left ventricular hypertrophy [2].
Let it be clear that having a high blood pressure is detrimental to health.
How do anabolic steroids affect blood pressure?
There are two sorts of studies we can have a look at to answer the question how anabolic steroids affect blood pressure. One sort of study is the prospective interventional trials. These, in essence, are the most reliable. You take a group of people and you give them an anabolic steroid and follow them over time to see what happens with their blood pressure. Additionally, you might include a control/placebo group to compare the results to (and if you randomize the subjects you got yourself a randomized-controlled trial). While such trials are definitely the best in terms of quality of evidence, they suffer from one major drawback: they don’t imitate the real-world usage properly since the dosages are lower than that used by most people who use anabolic steroids illicitly.
Having said that, let’s have a look at these trials. I’ve summed them up in the table below to provide a good overview:
| Study | AAS & dosage | Systolic BP | Diastolic BP |
| Giorgi et al. [3] | TE, 3.5 mg/kg bw for 12 weeks | ? (+10 mmHg)* | Unchanged |
| Zmuda et al. [4] | TE, 200 mg weekly for 3 weeks | “No clinically significant changes” | “No clinically significant changes” |
| Freed et al. [5] | Dbol, 10 mg or 25 mg daily for 6 weeks | ↑ (+9 mmHg)† | ↔ (+4 mmHg)† |
| Glazer et al. [6] | ND, 100 mg weekly for 6 weeks | ↔ (-5 mmHg)* | ↔ (-1 mmHg)* |
| Hartgens et al. [7] | ND, 200 mg weekly for 8 weeks | ↔ (+2 mmHg)† | ↔ (+3 mmHg)† |
| Kuipers et al. [8] | ND, 200 mg 1st week, 100 mg weekly for 7 weeks | ↔ (+1 mmHg)† | ↔ (-4 mmHg)† |
| Kuipers et al. [8] | ND, 200 mg 1st week, 100 mg weekly for 7 weeks | ↔ (+10 mmHg)† | ↔ (+3 mmHg)† |
Effect of anabolic steroids on blood pressure in prospective interventional trials. ↑ means a statistically significant increase, ? means no statistical testing was performed, * means compared to baseline, † means compared to the change in the placebo group. Abbreviations: BP, blood pressure; TE, testosterone enanthate; Dbol, methandienone; ND, nandrolone decanoate.
The only trial that demonstrated a statistically significant increase in (systolic) blood pressure was the one by Freed et al [5]. Herein, experienced weightlifters received 10 mg or 25 mg methandienone (dianabol) daily for 6 weeks in a double-blind placebo-controlled crossover fashion. Systolic blood pressure increased significantly by about 9 mmHg (estimated based on Figure 3). Diastolic blood pressure showed a slight increase of about 4 mmHg, but this was not statistically significant.
The other trials either didn’t perform statistical testing [3,4], or didn’t detect statistically significant changes either compared to baseline [6] or compared to the change in the placebo group [7, 8].
As you can also see when looking at the dosages: these were fairly low and cannot be called representative for anabolic steroid usage as regularly seen in bodybuilders and the likes. To get a better idea of that, one could look at prospective observational trials. In these trials AAS users are followed over time self-administering their own AAS cycle. Of course, these trials also have drawbacks. One being ‘polydrug usage’. Not all anabolic steroids might affect blood pressure equally, and when AAS users are stacking these it’s hard to say which anabolic steroid might’ve been responsible for it. Let alone the issue that there’s considerable probability that an AAS user is administering different anabolic steroids than he thinks he’s administering due to mislabeling. [9]. Additionally, AAS users might stack it with several other types of drugs such as growth hormone, thyroid hormone, beta-agonists, and nowadays the vast array of experimental performance-enhancing drugs of which usage is on the rise.
Let’s also briefly consider some prospective observational trials. Hartgens et al. observed the effects of self-administered AAS over a period of 8 weeks in a small group of strength athletes [10]. Prior to the trial, the subjects, on average, hadn’t used AAS for almost 8 months. The average dosage was relatively low at around 400 mg weekly, which does make me wonder how accurate this was. Either way, systolic blood pressure increased from 131 to 139 mmHg. The control group saw an increase from 129 to 134 mmHg. Thus the average change compared to the control group was +3 mmHg. A small 2 mmHg increase was observed in diastolic blood pressure, with no change in the control group. Regardless, the differences weren’t statistically significant.
If you assume that AAS can affect blood pressure, and that this is fully reversible after cessation of usage, you can also employ a slightly different study design. Namely, you could take a group of users while they are using AAS, measure their blood pressure, and then measure it again after some period of time when they’ve gone off the AAS. Exactly this is the type of approach that two other groups had employed [11, 12].
One of them evaluated three groups: sedentary subjects, nonusing bodybuilders, and AAS-using bodybuilders [11]. The AAS cycles lasted on average 8 weeks, and, unfortunately, the dosages cannot be accurately derived from the study. Nevertheless, they do appear on the low side of things. Right after their cycles, blood pressure measured 141/84 mmHg and 9 weeks after cessation of usage it was 140/83 mmHg. For reference, the nonusing bodybuilders had a blood pressure of 136/87 and the sedentary subjects of 139/85 mmHg.
Palatini et al. performed 24-hour blood pressure measurements in a small group of AAS users [12]. Cycles lasted on average 9 weeks and the dosage was about 500 mg weekly. Blood pressure was 128/83 mmHg at the end of their cycles versus 129/84 mmHg nearly 12 weeks after cessation.
In the HAARLEM trial, 100 anabolic steroid users were followed over time while they self-administered AAS [9]. Mean dosage, based on label information, was 898 mg per week, thus making their AAS cycle quite representative of common usage by bodybuilders. Measurements were taken before, during, as well as 3 months after the end of their cycle and 1 year after the start of their cycle. Unpublished data of this trial demonstrated an increase of 7 mmHg in systolic blood pressure and 3 mmHg in diastolic blood pressure during anabolic steroid use compared to baseline [DL Smit, personal communication]. These measurements returned to baseline after the cycle. Given the relatively large sample size of 100 AAS users, as well as the prospective observational nature of this trial, this is currently the best estimate to what extent AAS might affect blood pressure in dosages commonly employed by bodybuilders.
Taken together, one can cautiously conclude that supraphysiological dosages of AAS can transiently increase systolic blood pressure by roughly 5 to 10 mmHg during usage. It is hard to say to what extent this exacerbates cardiovascular risk. But we might be able to get some clues from other data in the scientific literature. I’ll cover that in the next article in which I discuss blood-pressure-lowering medication.
Conclusion
Having a high blood pressure is a silent killer. By far most people won’t feel it, but it dramatically increases the mortality due to coronary heart disease or stroke. Every 20 mmHg increase in systolic blood pressure beyond 115 mmHg, and every 10 mmHg increase in diastolic blood pressure beyond 75 mmHg, is associated with a doubling in mortality due to these causes. Additionally, hypertension over a prolonged period of time will lead to target organ damage. This not only affects the heart and the vasculature, but also other organs such as the brain, eyes and kidneys. Evidence from the literature indicates that anabolic steroid usage in high dosages has the potential to mildly increase blood pressure. A ballpark figure is about 5 to 10 mmHg for systolic blood pressure, and half of that for diastolic blood pressure. Of course, people might deviate from these ranges. This might or might not depend on dosage, the sorts of AAS used, as well as concurrent usage of ancillary drugs that might affect it. Fortunately, these increases are transient and reversible after cessation of usage.
In any case, hypertension can and should be treated when diagnosed. In my next article, I will discuss how blood pressure might be monitored by anabolic steroid users and which pitfalls to look out for while measuring. When to start treatment and which treatment modalities are available, and what beneficial effect (as well as potential harmful effects) they might incur, will also be described.
Image by Pera Detlic from Pixabay
References
- Prospective Studies Collaboration. “Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies.” The Lancet 360.9349 (2002): 1903-1913.
- Cohuet, G., and H. Struijker-Boudier. “Mechanisms of target organ damage caused by hypertension: therapeutic potential.” Pharmacology & therapeutics 111.1 (2006): 81-98.
- Giorgi, Anthony, Robert P. Weatherby, and Peter W. Murphy. “Muscular strength, body composition and health responses to the use of testosterone enanthate: a double blind study.” Journal of science and medicine in sport 2.4 (1999): 341-355.
- Zmuda, Joseph M., et al. “The effect of testosterone aromatization on high-density lipoprotein cholesterol level and postheparin lipolytic activity.” Metabolism 42.4 (1993): 446-450.
- Freed, D. L., et al. “Anabolic steroids in athletics: crossover double-blind trial on weightlifters.” Br Med J 2.5969 (1975): 471-473.
- Glazer, Gary, and Anthony L. Suchman. “Lack of demonstrated effect of nandrolone on serum lipids.” Metabolism 43.2 (1994): 204-210.
- Hartgens, F., E. C. Cheriex, and H. Kuipers. “Prospective echocardiographic assessment of androgenic-anabolic steroids effects on cardiac structure and function in strength athletes.” International journal of sports medicine 24.05 (2003): 344-351.
- Kuipers, H., et al. “Influence of anabolic steroids on body composition, blood pressure, lipid profile and liver functions in body builders.” International journal of sports medicine 12.04 (1991): 413-418.
- Smit, Diederik L., et al. “Baseline characteristics of the HAARLEM study: 100 male amateur athletes using anabolic androgenic steroids.” Scandinavian journal of medicine & science in sports 30.3 (2020): 531-539.
- Hartgens, F., E. C. Cheriex, and H. Kuipers. “Prospective echocardiographic assessment of androgenic-anabolic steroids effects on cardiac structure and function in strength athletes.” International journal of sports medicine 24.05 (2003): 344-351.
- De Piccoli, B., et al. “Anabolic steroid use in body builders: an echocardiographic study of left ventricle morphology and function.” International journal of sports medicine 12.04 (1991): 408-412.
- Palatini, Paolo, et al. “Cardiovascular effects of anabolic steroids in weight‐trained subjects.” The Journal of Clinical Pharmacology 36.12 (1996): 1132-1140.
About the author
Peter Bond is a scientific author with publications on anabolic steroids, the regulation of an important molecular pathway of muscle growth (mTORC1), and the dietary supplement phosphatidic acid. He is the author of several books in Dutch and English, including Book on Steroids and Bond's Dietary Supplements.

Leave a Reply
You must be logged in to post a comment.