
Estimated reading time: 7 minutes
Table of contents
Introduction
1-Testosterone (17β-hydroxy-5α-androst-1-en-3-one; 17-hydroxyandrost-1-en-3-one; 1-Testo; DHB or dihydroboldenone) is non-aromatizable and non-5α-reducible, a member of the androst-1-ene-3-one (“1-ene”) class of anabolic-androgenic steroids (AAS), like metenolone (Primo) and stenbolone. As a 1-ene, 1-testosterone is an AAS whose anabolic features predominate over its androgenic (masculinizing, suppressive, male-pattern balding, etc.) features.
1-Testosterone interestingly metabolizes primarily to dihydrotestosterone (5α-dihydrotestosterone; DHT) despite being non-5α-reducible, and is particularly hepatotoxic – unusual for an injectable AAS – both directly, with evidence of its increasing liver size in animal data, and indirectly, since industrial grade solvents like guaiacol, a hazardous chemical and likely carcinogen, are typically used by manufacturers and chemists to hold 1-testosterone in solution.
1-Testosterone’s particular intolerability and contentious anabolic effects – being regarded by one faction of loyalists as a “Tren lite,” versus another faction of detractors that regard it as basically Primo [of the same class of 1-enes] but with post-injection pain (PIP) and C-reactive protein (CRP) elevations, rank it among the less useful AAS category for bodybuilders.
Increases DHT
Metabolism to Dihydrotestosterone via Non-5α-Reductase Pathway
1-Test produces as urine metabolites:
- 5α-dihydrotestosterone (DHT)
- 5α-androst-1-ene-3α,17β-diol
- 5α-androst-1-ene-3,17-dione
- 5α-androst-1-ene-3α-ol-17-one
- 1-epitestosterone
[1]
Potentially 1,2-dihydrogenated by an Unidentified Enzyme
1-Testosterone produces DHT as its primary metabolite despite being non-5α-reducible. [1]. There are only imperfect explanations for its yielding DHT as a metabolite. Peter Bond indicated by email that he was surprised by Fragkaki’s identification of DHT as the primary metabolite of 1-testosterone since it suggests that some heretofore unidentified enzyme is responsible for 1,2-dihydrogenation of this androst-1-ene-3-one. [2].
Increases Estrone (E1)
Potential 17β-HSD1 Inhibitor
17β-HSD1 Inhibitors Increase DHT and Estrone (E1) and Decrease Estradiol (E2)
It is this author’s working hypothesis that 1-testosterone may act as a 17β-HSD1 inhibitor, thereby increasing DHT and E1, and decreasing E2.
The structurally similar DHT is a known, albeit weak, 17β-HSD1 inhibitor. Figure 2. For the androgens DHT or testosterone (T) to become a good ligand for this enzyme, they must more closely mimic the planar shape of the A-ring of E2 and provide a large hydrophobic core. [3]. 1-Testosterone meets both requirements, differing from DHT by a C-1,2 double bond, giving it a more estrogen-like A-ring and from testosterone by its large hydrophobic 5α-androstene backbone. Such 5α-androstenes have 173-fold greater affinity for the AR than their β-oriented counterpart, e.g., DHT has increased flatness and is less bulky versus T to fit into the hydrophobic cavity of the binding site on the AR. [4].
![Figure 2: 17β-HSD1 binding by DHT: (A) - (E) DHT/17β-HSD1 complex 3D crystallization and docking data from [3]](https://thinksteroids.com/wp-content/uploads/2024/12/17β-HSD1-binding-DHT.png)
The 17β-HSD1 enzyme is highly expressed in adipose tissue and prostate in men. [5]. Its function is to catalyze reduction of the weak estrogen E1 to the strong estrogen E2, and its expression positively correlates to E1 activation & E2 levels. [5]. Inhibition of 17β-HSD1 reduces E2, though abolition (to 0.00 pg/mL) would require inhibition of aromatase & sulfatase, also. [5].
17β-HSD1 catalyzes the last step in the biosynthesis of active estrogens (E2 & 5-androstene-3β,17β-diol) while E1 is its primary substrate, the enzyme has some activity for non-estrogenic substrates. DHT is a non-cognate substrate for 17β-HSD1 and binds it similarly to E2, in similar orientation, but with some differences to accommodate additional bulk (e.g., the C-19 methyl group). The complex (17β-HSD1-DHT) has well defined steroid-binding sites and the known Km value for DHT is 17 times higher than for E2 (so it takes more DHT to saturate the enzyme) and the specific activity 26 times lower. [6].
In conclusion, 1-testosterone must be, if inhibitory of 17β-HSD1, weakly so – certainly not with sufficient specificity to be marketed for clinical use in breast cancer treatment. While the literature cited here suggests that C19 androgens are not highly potent & selective 17β-HSD1 inhibitors – so as to enable good binding and a high reaction rate – the androgens are active ligands for the enzyme. In order to maximize binding, an inhibitor designed to be specific for 17β-HSD1 must mimic the planar shape of the A-ring of E2 and maintain a large hydrophobic core that can interact with the steroid binding site of the enzyme. [6]. 1-Testosterone’s more estrogen-like A-ring and large hydrophobic 5α-androstene backbone match with these requirements making it one C19 androgen that is theoretically more potently inhibitory of 17β-HSD1 than DHT.
Hepatotoxic
In one rodent study, parenteral (intramuscular) injection of 1-testosterone was associated with liver growth, indicative of an increased hepatic risk from 1-testosterone itself. [7]. Extrapolating from the dose used of 1 mg/kg bodyweight (rodent) for 12 days by applying a human equivalence multiplier, the study’s human dosing equivalence is 112 mg weekly for a man weighing 100 kg (220 lb). [8].
Anecdotally, blood test results demonstrate an increase in C-reactive protein (CRP), that reflects inflammation, in common 1-testosterone injectable preparations. It is difficult to keep in solution, so the product frequently “crashes” and it is known to cause unbearable “PIP,” or post-injection pain.
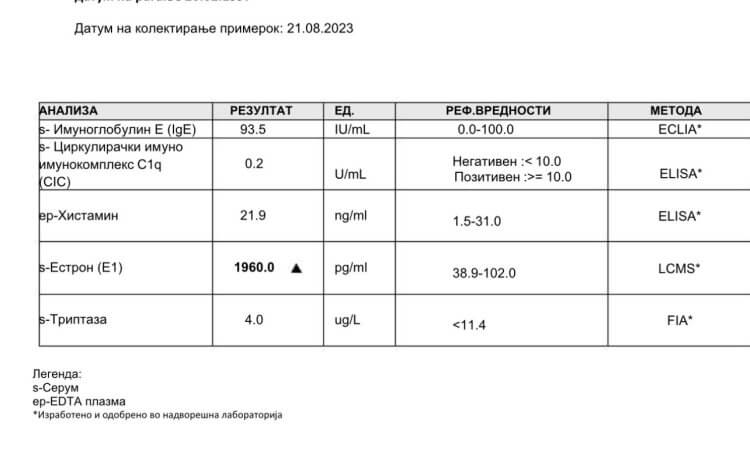
Carcinogenic
Guaiacol is a commonly used solvent that is used by black market AAS manufacturers to hold 1-testosterone in solution. Guaiacol is a chemical irritant, a disinfectant, and cough expectorant. [9]. It is classified as a hazardous chemical, and according to the World Health Organization’s International Agency for Research on cancer, guaiacol is probably carcinogenic to humans. [10].
Most remarkably, however, is the 1-testosterone record from the European Chemicals Agency (ECHA) that indicates that 1-testosterone itself may cause cancer:
Conclusion
1-Testosterone is a 1-ene, attenuated androgen, of the same class as stenbolone and metenolone (Primo), regarded as mild anabolics. It is remarkably intolerable, associated with “crashing” out of solution, the use of guaiacol (a hazardous chemical irritant, disinfectant, and cough expectorant, that is likely carcinogenic), and indeed inherent carcinogenic risk, hepato-, and generalized toxicity. Its most interesting feature is its potential, albeit fairly nonselective, 17β-HSD1 inhibition. For those particularly sensitive to potent estrogens like E2 but that fare better on drugs like boldenone (EQ) – and this is true especially for women, who empirically report good tolerability for drugs that principally aromatize to E1 [perhaps due to different estrogen profiles versus men, and greater endogenous E1 levels] – 1-Testosterone might be tempting to use for its mild estrogenic effects, even modulating influence on estrogens.
References
[1] Fragkaki, A. G., Angelis, Y. S., Tsantili-Kakoulidou, A., Koupparis, M., & Georgakopoulos, C. (2009). Schemes of metabolic patterns of anabolic androgenic steroids for the estimation of metabolites of designer steroids in human urine. The Journal of Steroid Biochemistry and Molecular Biology, 115(1-2), 44–61. doi:10.1016/j.jsbmb.2009.02.016
[2] Bond, Peter. “Re: 1-Test & its surprising metabolic fate.” Received by Cormac Mannion (Type-IIx). 2 April 2022.
[3] He, W., Gauri, M., Li, T., Wang, R., & Lin, S.-X. (2016). Current knowledge of the multifunctional 17β-hydroxysteroid dehydrogenase type 1 (HSD17B1). Gene, 588(1), 54–61. doi:10.1016/j.gene.2016.04.031
[4] Liao, S., Hung, S. C., Tymoczko, J. L., & Liang, T. (1976). Active Forms and Biodynamics of the Androgen-Receptor in Various Target Tissues. Steroid Hormone Action and Cancer, 139–151. doi:10.1007/978-1-4684-2601-4_12
[5] Thomas MP, Potter BV. The structural biology of oestrogen metabolism. J Steroid Biochem Mol Biol. 2013;137:27-49. doi:10.1016/j.jsbmb.2012.12.014
[6] Lin, S. X., Han, Q., Azzi, A., Zhu, D.-W., Gongloff, A., & Campbell, R. L. (1999). 3D-structure of human estrogenic 17β-HSD1: binding with various steroids. The Journal of Steroid Biochemistry and Molecular Biology, 69(1-6), 425–429. doi:10.1016/s0960-0760(99)00062-x
[7] Friedel, A., Geyer, H., Kamber, M., Laudenbach-Leschowsky, U., Schänzer, W., Thevis, M., … Diel, P. (2006). 17β-Hydroxy-5alpha-androst-1-en-3-one (1-testosterone) is a potent androgen with anabolic properties. Toxicology Letters, 165(2), 149–155. doi:10.1016/j.toxlet.2006.03.001
[8] Reagan-Shaw, S., Nihal, M., & Ahmad, N. (2008). Dose translation from animal to human studies revisited. The FASEB Journal, 22(3), 659–661. doi:10.1096/fj.07-9574lsf
[9] DrugBank 6.0: the DrugBank Knowledgebase for 2024. “Guaiacol, Record: DB11359” DrugBank, https://www.drugbank.ca/drugs/DB11359. Accessed 14 November 2024.
[10] IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). [link redacted], p. S7 177 (1987).
[11] ECHA. Notified classification and labelling acording to CLP criteria.5α-Androst-1-en-17β-ol-3-one (EC: 636-242-4), https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/168879. Accessed 14 November 2024.
About the author
Type-IIx is a physique coach, author, and researcher. Bolus: A Practical Guide and Reference for recombinant Human Growth Hormone Use will be his first published textbook, anticipated for release in early 2023. Ampouletude.com will be Type-IIx's base of operations for coaching services and publications. Type-IIx is proud to be a contributing writer to MesoRx, his home forum, where he is a regular poster.
Leave a Reply
You must be logged in to post a comment.