
Estimated reading time: 12 minutes
Table of contents
- Introduction
- ✓ Estrogens and Lipid management
- ✓ Estrogens and Adipose Tissue and Metabolism
- ✓ Estrogens and Skeletal Muscle
- Estrogens and Collagen Metabolism
- ? Estrogens and Sexual Function
- ✓ Estrogens and Renal Function
- ? Estrogens and the Cardiovascular System
- ✖ Estrogens and the Immune System
- ↑ Fluid Retention (Edema)
- Conclusion
- References
Introduction
The endogenous functions of the classical female sex hormones, that include estrone (E₁), estradiol (E₂), and estriol (E₃), are well described in that sex. This article is directed at estrogen functions in men, that are less well described – and certainly misunderstood by most bodybuilders. This article is intended to clarify the positive and negative aspects of estrogens for male bodybuilders, those using anabolic-androgenic steroids (AAS), and men generally.
In men, testes produce only about ~20% of circulating estrogens. The remainder come from local production by adipose, brain, skin, and bone tissues, which contain aromatase (T ⇒ E₂). [1]. Peripheral blood T concentrations in men of ~20 nM (495) are at least two orders of magnitude greater than E₂ concentrations (30 – 200 pM) (125, 397, 495, 607). [1].
While exogenous estrogens cause male reproductive pathologies [1], endogenous estrogens are vital for male sexual functioning. In part, this is due to a dramatic increase in SHBG (reducing T bioavailability). [8].
✓ Estrogens and Lipid management
In order to discuss the benefits to lipid management vis-à-vis estrogens in a manner most relevant to the readership, to wit, AAS-using bodybuilders, let us first discuss the well-known deleterious impact of AAS on lipids.
AAS Effects on Lipids are modulated via:
(a) ↑ Hepatic triglyceride lipase activity (HTGLA), thereby reducing high-density lipoproteins (↓HDL-C), and
(b) ↑Apo B, therefore ↑LDL-C.
Firstly, with respect to (a): hepatic lipase (HL) is a liver-secreted enzyme that liberates fatty acids from triacylglycerol and phospholipids that are part of lipoproteins, including high-density lipoproteins (HDL). AAS, by increasing its activity (HTGLA), induce a shift from larger HDL₂ to smaller HDL₃ particles, susceptible to yet further breakdown, and thereby reduce HDL. [14].
Secondly, with respect to (b): Apo B is strongly associated with very-low density lipoproteins (VLDL; a most atherosclerotic particle class) and gives insight into LDL actually in bloodstream circulation – perhaps by AAS increasing liver secretion of these lipoproteins. [16].
Parenteral (i.m.) vs. Oral (p.o.) AAS Effects on Lipids
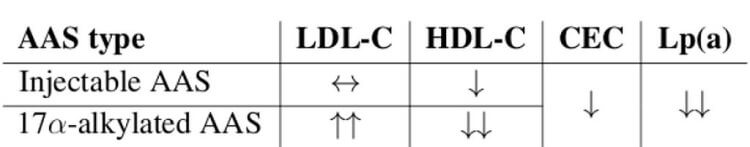
Dyslipidemia
Dyslipidemia is marked by ↑LDL-C and ↓HDL-C.
Mechanisms of Dyslipidemia
HDL-C
The nonaromatizable 17α-alkylated AAS (17AAs), because they are either nonaromatizable or resistant to aromatization and therefore lack estrogen benefits to lipids (estrogens ↓HTGLA), and because they are metabolized primarily in liver, having greater effects on hepatic proteins (e.g., HL), are more dyslipidemic and atherosclerotic than parenteral androgens (especially T). [15].
LDL-C
17AAs may ↑Apo B in particular, [16] – that is associated with VLDL and LDL actually in bloodstream circulation – perhaps by ↑ liver secretion of these lipoproteins.
Estrogens produced by aromatizing androgen, particularly T, benefit lipids by reducing HTGLA, provoking a net flux towards larger HDL₂ particles, thereby ameliorating dyslipidemia and atherosclerosis.
✓ Estrogens and Adipose Tissue and Metabolism
Researchers use estrogen receptor knock-out (ERKO) male mice to study the effects of E₂ per se without any confounding influence from T. ERKO male mice possess adipose tissue (AT; fat tissue) depots that are 100% larger by 9 – 12 months (approximately middle-age in humans). This increase in fat tissue reflects both adipocyte (fat cell) hyperplasia and hypertrophy (281) and is accompanied by glucose intolerance and insulin resistance (IR). ERαKO male mice, those with the ER-α knocked-out but with the ER-β intact, showed ↑AT inflammation, adipocyte size and impaired glucose tolerance versus normal male mice.
Glucose metabolism per kg of muscle is 45% higher in women (756) and this is likely ER-α-mediated. [1]. In men, T’s beneficial metabolic effects are mediated more by its aromatic product (E₂) than by androgen (E₂ > T in ↓AT accrual). Approximately 15% of circulating estrogens in men derive from testicular synthesis and secretion (in the Leydig cell) and the remainder comes from peripheral aromatase activity. [9].
Practically, for men using supraphysiologic doses of aromatizable androgen (e.g., T, nandrolone [Deca, NPP], boldenone [EQ], trestolone [MENT], metandienone [Dianabol]), this means that that the extra-testicular sites of aromatase, particularly fat cells, are predominately responsible for the effects associated with estrogens (estrogenicity). For bodybuilders that associate estrogens with unfavorable changes to fat mass, they’re not wrong per se. This is because while estrogens reduce fat mass, they do redistribute fat mass to a more feminine pattern – especially to the buttocks and hips – rather than to the abdominal depot, a more male pattern of fat distribution.
✓ Estrogens and Skeletal Muscle
ER-α and -β are expressed in human skeletal muscle (737) ubiquitiously, in myofibers, endothelial cells and satellite cells (346, 737, 738). Endurance training increases ER-α and -β in human skeletal muscle, suggesting their expression is altered by functional demands in muscle (738). [1].
Whereas ER-α primarily regulates blood lipids and bone remodeling, ER- β is thought to mediate remodeling in muscle tissue.
Estradiol (E₂) is a “potent” ligand for ER-β. Its rate of dissociation (κd) for this receptor is 2.08 nM in humans. [17]. Its rate of dissociation (κd) for ER-α, by comparison, is a mere 0.24 nM in humans. [17]. This may explain much of its anabolic effect in muscle.
Estrogens are constituents of steroid regimens administered to castrated male cattle to stimulate muscle growth and improve carcass quality (316, 337), indicating that estrogens have anabolic effects on male muscle mass. [7]. It is important to note key differences between bovid and man, however. In bovid, exogenous E₂ dose-dependently augments IGF-I, but in man, E₂’s effect on IGF-I is parabolic, subject to an inverse-U shape when plotting dose (i.e., blood concentration) on the abscissa (x-axis) versus response (IGF-I) on the ordinate (y-axis) axes. This is because estrogens (e.g., E₂) decrease IGF-I bioavailability by increasing IGFBP-1. [7].
Estrogen replacement (ERT; HRT) appears to facilitate skeletal muscle growth in postmenopausal women (644), possibly through effects on muscle satellite cells. [1]. Post-menopause, women experience a chronic decline in estrogen levels. This is associated with a reduction in sensitivity to anabolic stimuli (e.g, resistance training,) that is reversible by ERT. While ERT may actually decrease basal muscle protein synthesis, it increases post-resistance training growth, i.e., anabolic response. [2].
Estrogens and Collagen Metabolism
Estrogen has a dramatic effect on musculoskeletal function given its roles in the development, maturation, and aging of bone, tendon, and ligament.
Estrogen improves muscle mass and strength and increases collagen content of connective tissues. However, unlike in bone and muscle where estrogen improves function, in tendons and ligaments, estrogen ↓stiffness, therefore ↓performance and power and ↑ligament injury risk. [2]
Whereas this article is focused on estrogens, a forthcoming article written by this author related to AAS and collagen, joints, and bone (per-compound) will delve into the effects of particular aromatizable (e.g., testosterone, nandrolone, metandienone) and nonaromatizable (e.g., stanozolol, oxandrolone) androgens, rhGH, and other anabolic agents on connective tissues including tendons, ligaments, and bone. There are per-class and per-compound differences in these effects. While some AAS and other anabolic agents benefit connective tissues; others harm it.
✓ Bone
Although androgens have significant effects on male bone (653), estrogens are more important for bone growth and maintenance (440, 589). [6]. E₂ is essential for normal bone mineralization, mass, and turnover in men (648, 649). [6].
Aromatization of T ⇒ E₂ is essential for T (T + E₂ > T) effects on bone (loss + BMD). E₂ is clearly required for normal bone growth and maintenance in men (104) and E₂ mediates some T effects on bone homeostasis (353). [1]. Estrogen’s ultimate action on the skeleton is to decrease bone remodeling and bone resorption while maintaining bone formation. [6].
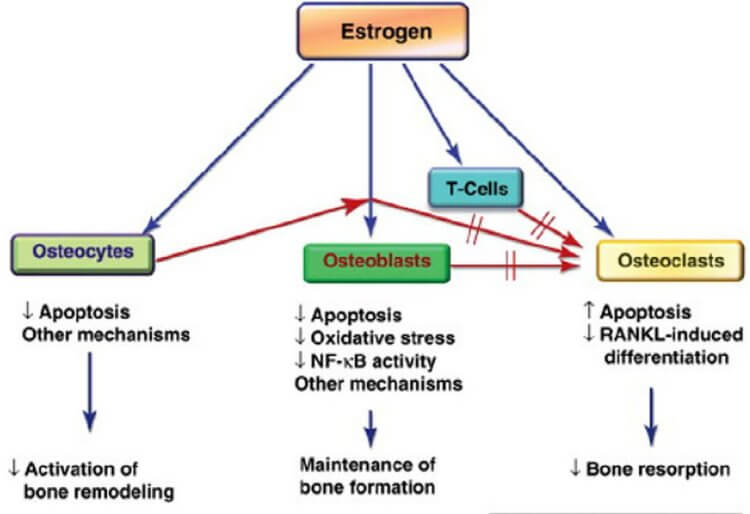
✖ Tendon and Ligament
Tendon is connective tissue connecting bone to muscle. Ligament is connective tissue connecting bone to bone.
Estrogen partly increases collagen content of tendon by an indirect effect on IGF-I. Estrogens directly modulate both IGF-I and IGFBPs (Hansen, et al., 2009b), and therefore can, via IGF-I, affect collagen content through an increased collagen protein synthesis via production of the LARP6 (La-related protein 6) (Blackstone, et al., 2014). [20]. LARP6 is a binding protein that is increased by IGF-I, that directly binds to type I collagen mRNA, and specifically increases translation of type I collagen. [20].
In both tendon and ligament, the dry weight composition is primarily collagen – between 60 – 85% in tendon and ~75% in ligament. Of this collagen, most is type I: 60% in tendon and up to 85% in ligament.
Stiffness of ligament a Good Thing (✓), as stiffness is beneficial to maintain joint stability and ↓injury risk in these sinews that attach bones to other bones. Conversely, since tendon connects stiff bone to compliant muscle, a stiffer tendon is not always beneficial.
Stiffness of tendon is mixed – stiffer tendons benefit performance but also increase injury risk. In terms of performance (e.g., strength, power, sprint), increasing tendon stiffness transmits muscle forces faster to bone, thereby ↑performance; however, this interest must be balanced against the potential for strain concentration within the muscle, causing rupture. When a muscle attached to a lax tendon contracts, the tendon stretches pliably while the muscle shortens. A stiff tendon, on the other hand, does not stretch; rather, it is forced to lengthen while contracting, causing eccentric forces. This means that in a muscle that is attached to a stiff tendon, greater eccentric load for a given movement increases injury risk.
Estrogen decreases both tendon and ligament stiffness, thereby reducing performance and increasing the risk of ligament rupture.
? Estrogens and Sexual Function
ER-α signaling in men supports:
✓ Efferent ductules and epididymal functions. [1].
✓ Ion transport and H₂O reabsorption necessary to support normal sperm (male reproduction). [1].
✖ FSH and LH concentrations. [18].
Like E₂ vis-à-vis IGF-I, estrogen’s effect on sexual function is parabolic and subject to an inverse-U shape when plotting dose (i.e., blood concentration) on the abscissa (x-axis) versus sexual function (e.g., libido, erectile quality, fertility) on the ordinate (y-axis) axes. This is because the concentrations at which E₂ supports efferent ductules, epididymal functioning, and sperm quality and motility is low, and when estrogens are high (as in the case of supraphysiologic T doses), FSH and LH concentrations and the full cascade of hypothalamo-pituitary gonadal functioning begins to become negatively regulated. Estradiol is a more potently suppressive hormone than T. [7].
✓ Estrogens and Renal Function
Estrogens have positive effects on pancreatic β-cell function and diabetes mellitus incidence that are mediated in part by estrogen effects on β-cell apoptosis, β-cell insulin content, insulin gene expression, and insulin release. [1].
? Estrogens and the Cardiovascular System
It is eminently unclear whether estrogen serves a positive or negative effect on the cardiovascular system (309, 390, 592). [1]. While some protective effects of T are mediated indirectly through E₂ (488), lower serum T is more strongly associated with higher risks of death from cardiovascular disease in men than serum E₂ changes (309, 390, 592). [1]. In contrast, higher serum E₂ levels were reported in men with coronary disease (548) and sudden cardiac arrest (483). [1].
✖ Estrogens and the Immune System
In contrast to females, E₂ actually inhibits wound healing in males through ER-α (243). [1].
↑ Fluid Retention (Edema)
E₂ influences fluid retention by action on the CNS (regulating thirst, fluid and sodium intake) and RAAS (principally by acting on aldosterone). [11].
Conclusion
Bodybuilders in their off-season benefit from increased E₂ due to improved response to anabolic stimuli and repair (effects on muscle damage), but must contend with fluid retention and a more female fat distribution, to the hips and buttocks. Aromatization increases IGF-I, and therefore total body size, but blood E₂ levels must be optimized rather than maximized due to the parabolic, inverse-U shape to the E₂/IGF-I curve. Considerations about training loading must be considered, however, due to the deleterious effects of estrogen on tendon and ligament mechanics (reduced stiffness). Overzealous use of aromatase inhibitor (AI) drugs may have unintended consequences via reducing estrogens, particularly on bone metabolism, lipid management, and muscle size, particularly the anabolic response to training.
References
[1] Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in Male Physiology. Physiol Rev. 2017 Jul 1;97(3):995-1043. doi:10.1152/physrev.00018.2016.
[2] Chidi-Ogbolu N, Baar K. Effect of Estrogen on Musculoskeletal Performance and Injury Risk. Front Physiol. 2019;9:1834. Published 2019 Jan 15. doi:10.3389/fphys.2018.01834
[3] Zhang Y, Stefanovic B. mTORC1 phosphorylates LARP6 to stimulate type I collagen expression. Sci Rep. 2017 Jan 23;7:41173. doi:10.1038/srep41173.
[4] Kim, J. H., Cho, H. T., and Kim, Y. J. (2014). The role of estrogen in adipose tissue metabolism: insights into glucose homeostasis regulation [Review]. Endocrine Journal, 61(11), 1055–1067. doi:10.1507/endocrj.ej14-0262
[5] Eyster, K. M. (Ed.). (2016). Estrogen Receptors. Methods in Molecular Biology. doi:10.1007/978-1-4939-3127-9
[6] Cauley, J. A. (2015). Estrogen and bone health in men and women. Steroids, 99, 11–15. doi:10.1 016/j.steroids.2014.12.010
[7] Veldhuis, J. D., and Bowers, C. Y. (2003). Human GH pulsatility: An ensemble property regulated by age and gender. Journal of Endocrinological Investigation, 26(9), 799–813. doi:10.1007/bf03345229
[8] Damewood MD, Bellantoni JJ, Bachorik PS, Kimball AW Jr, Rock JA. Exogenous estrogen effect on lipid/lipoprotein cholesterol in transsexual males. J Endocrinol Invest. 1989 Jul-Aug;12(7):449-54. doi:10.1007/BF03350728.
[9] Rubinow KB. Estrogens and Body Weight Regulation in Men. Adv Exp Med Biol. 2017;1043:285-313. doi:10.1007/978-3-319-70178-3_14
[10] Stachenfeld, N. S. (2014). Hormonal Changes During Menopause and the Impact on Fluid Regulation. Reproductive Sciences, 21(5), 555–561. doi:10.1177/1933719113518992
[11] Curtis, K. S. (2015). Estradiol and osmolality: Behavioral responses and central pathways. Physiology and Behavior, 152, 422–430. doi:10.1016/j.physbeh.2015.06.017
[12] Warner M, Fan X, Strom A, Wu W, Gustafsson JÅ. 25 years of ERβ: a personal journey. J Mol Endocrinol. 2021 Oct 15;68(1):R1-R9. doi: 10.1530/JME-21-0121
[13] Kley, H. K., Deselaers, T., Peerenboom, H., and Kruskemer, H. L. (1980). Enhanced Conversion of Androstenedione to Estrogens in Obese Males*. The Journal of Clinical Endocrinology and Metabolism, 51(5), 1128–1132. doi:10.1210/jcem-51-5-1128
[14] Thompson, P. D. (1989). Contrasting Effects of Testosterone and Stanozolol on Serum Lipoprotein Levels. JAMA: The Journal of the American Medical Association, 261(8), 1165. doi:10.1001/jama.1989.03420080085036
[15] Friedl, K. E., Hannan, C. J., Jones, R. E., and Plymate, S. R. (1990). High-density lipoprotein cholesterol is not decreased if an aromatizable androgen is administered. Metabolism, 39(1), 69–74. doi:10.1016/0026-0495(90)90150-b
[16] Hartgens, F. (2004). Effects of androgenic-anabolic steroids on apolipoproteins and lipoprotein (a). British Journal of Sports Medicine, 38(3), 253–259. doi:10.1136/bjsm.2003.000199
[17] Perkins, M. S., Louw-du Toit, R., and Africander, D. (2017). A comparative characterization of estrogens used in hormone therapy via estrogen receptor (ER)-α and -β. The Journal of Steroid Biochemistry and Molecular Biology, 174, 27–39. doi:10.1016/j.jsbmb.2017.07.022
[18] Bond, P. Article: Regulation of Testosterone Production. Aug 2021. Source: https:/ /thinksteroids.com/articles/regulation-of-testosterone-production/
[19] Bond P, Smit DL, de Ronde W. Anabolic-androgenic steroids: How do they work and what are the risks? Front Endocrinol (Lausanne). 2022 Dec 19;13:1059473. doi: 10.3389/fendo.2022.1059473. PMID: 36644692; PMCID: PMC9837614.
About the author
Type-IIx is a physique coach, author, and researcher. Bolus: A Practical Guide and Reference for recombinant Human Growth Hormone Use will be his first published textbook, anticipated for release in early 2023. Ampouletude.com will be Type-IIx's base of operations for coaching services and publications. Type-IIx is proud to be a contributing writer to MesoRx, his home forum, where he is a regular poster.
Leave a Reply
You must be logged in to post a comment.