
Estimated reading time: 9 minutes
Table of contents
Introduction
Fluoxymesterone (Halotestin®; Androxy®; “Halo”), a member of the androst-4-ene-3-one class of androgens, an11β-hydroxylated orally administered AAS that is 5α/β-reducible and relatively nonestrogenic. is regarded as the penultimate androgen: a “hardening,” or “peaking” drug for competitive bodybuilders that is used late in the preparatory phase pre-contest.
Firstly, Halo is reportedly potently stimulatory of strength and aggression that is likely associated with enhanced central motor command and neural drive a la testosterone, in the case of moving from a hypo- to eu- gonadal state. [1]. Because of this notoriety for increasing aggression, a nocebo effect, a phenomenon by which the expectation of unfavorable interventional outcomes brings them about – the evil cousin of the placebo effect – applies to the use of Halo with respect to mood, behavior, and impulse control.
Secondly, it is expensive and rare. Demand for this drug is high and extends beyond the bodybuilding market to include strongmen and powerlifters to peak for their contests as well, and it is used clinically for oral delivery to treat endocrine disorders. Famously, President John F. Kennedy, Jr. (JFK) was prescribed this drug to treat complicated Addison’s Disease. [2]. His reputation for sexual prowess was legendary, and his infidelities may have been partly traceable to his use of the highly androgenic Halo!
Here in the third installment of an eleven-part series, the unique effects of Halo will be discussed thoroughly and concisely so that the reader can come to appreciate the subtle unique aspects that make Halo interesting.
Fluoxymesterone’s Unique Features
- Anticatabolic effects by antagonizing glucocorticoid receptor (GR). [3].
- Antiadopogenic (fat-mass reductive) effects likely by:
- This same glucocorticoid GR antagonism, and
- Androgenic potency. [3]. [4].
Anticatabolic
Fluoxymesterone is structurally unique among the universe of commercially available AAS since it is characterized by the placement of an α-fluorine at C-9 & a β-hydroxy group at C-11 which give it a structural similarity to the muscle catabolic glucocorticoids (e.g., cortisol, dexamethasone). [5].
Fluoxymesterone antagonizes Glucocorticoid Receptor
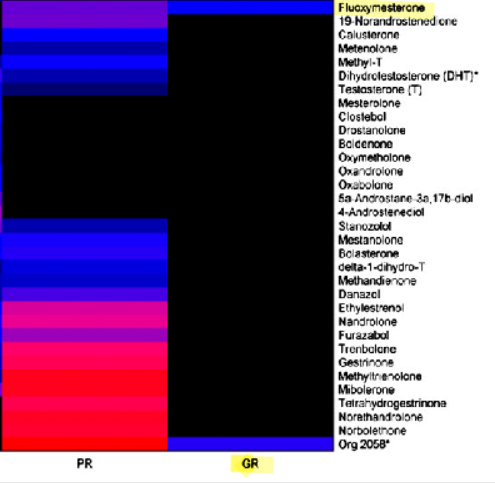
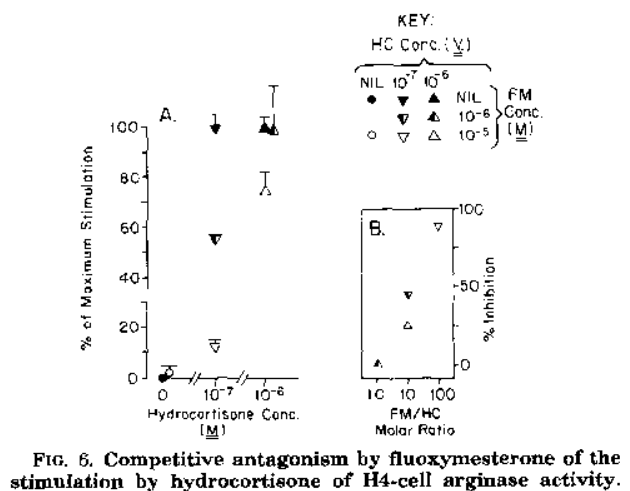
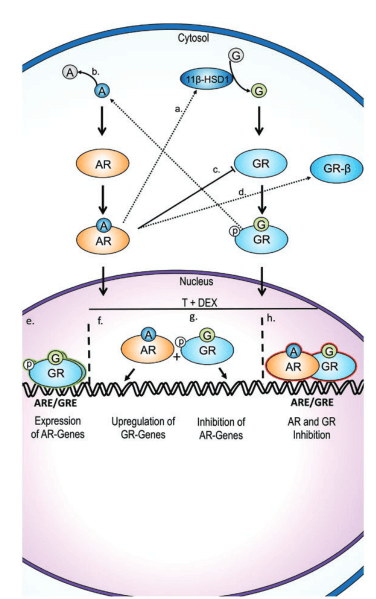
Fluoxymesterone exerts antiglucorticoid effects by binding weakly to the glucocorticoid receptor (GR) as an antagonist. [6]. [3]. Figures 1 & 2. Since this competitive mode of binding is indicated by assays from various tissues (i.e., GR CALUX ® mammalian bioassay and H-4 glial cells), this is likely systemic, unlike trenbolone’s skeletal muscle tissue-specific mode of GR modulation.
Antiadopogenic: Fat Mass Reductive
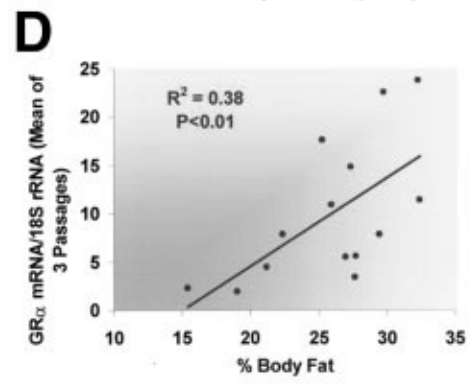
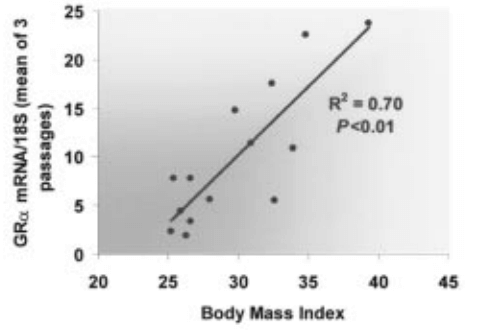
GR expression is associated with body fat % and body-mass index (BMI)
In human skeletal muscle, there is a “modest” correlation (R² = 0.38) between GR expression and body fat %, and a “more significant” correlation (R² = 0.70) between GR expression and body-mass index (BMI) that is indicative of an interplay between glucocorticoid modulation by fluoxymesterone and adipose tissue regulation. [7].
Fat mass reductions are associated with fluoxymesterone’s androgenic potency
Vida writes, in his treatise, Androgens and Anabolic Agents – where the 3-dimensional shape of the androgen receptor (AR) was first drawn (with shocking accuracy later confirmed by structure-activity relationship modeling – in a section titled, Compounds Displaying Increased Androgenic Activity Coupled With An Even Larger Increase in the Anabolic Activity:
[Halotestin] was prepared in the hope of obtaining a large increase in the androgenic and anabolic activities similar to that observed in the corticoid series by 9alpha-fluro 11beta-hydroxy substitution. The compound was found to possess 9.5 times the androgenic and 20 times the anabolic activity of 17alpha-methyltestosterone (37).
In spite of the fact that fluoxymesterone has a favorable anabolic-androgenic ratio, due to the strong androgenic effect the compound has been mainly used as an androgen, for example, as a highly active oral substitute for testosterone in hypogonadic condition (267) or as a phallotropic androgen (268). [4]. [8].
DHT (5α-dihydrotestosterone) is the classical “androgen,” potently androgenic but only weakly anabolic due to its extensive metabolism by 3α-HSD. [9]. Used as the study compound, DHT [comparable in its androgenic potency to fluoxymesterone] was shown by Gupta, Bhasin, et al. to:
- Dose-dependently inhibit lipid accumulation [reduce fat storage]
- Enhance p-ratio by enhancing insulin sensitivity, leptin sensitivity, and fat mass reduction
- Commit preadipocytes to a myogenic (i.e., muscle) rather than adipogenic (i.e., fat) lineage, and
- Induce lipolysis. [10].
But there is more we don’t know than do know about its antiadipogenic mechanisms!
A point must be made about the interaction between fluoxymesterone’s glucocorticoid modulation and fat mass reduction, however. By inference, we might inductively reason from the observation that we know – because we see with our eyes – that Halo is particularly reductive of fat mass. However, we must take care to not pigeonhole assumptions about cortisol and fat mass into the case of Halo’s GR antagonism. This is because:
- Fluoxymesterone also competitively inhibits 11β-HSD2 [like oxymetholone and testosterone] that controls the oxidation of cortisol, inactivating it, thereby increasing active cortisol’s effects, increasing serum & free cortisol. [11]. [12]. The nature of 11β-HSD2’s regulation of metabolic tissues including adipose and skeletal muscle is not well understood by contrast with its counterpart, 11β-HSD1, which converts cortisol to inactive cortisone. [13]. Therefore, our understanding of how this prong of fluoxymesterone action on adipose tissues is incomplete due to a dearth in the literature.
- Crosstalk between AR and GR vis-à-vis glucocorticoids and androgens is context- and tissue- dependent. [14]. Fluoxymesterone activity therefore regulates transcriptional output differently in different metabolic tissues, including between fat depots (e.g., white or brown fat) and skeletal muscle.
- The nature of crosstalk between AR and GR is poorly defined. [15]. Figure 5.
Conclusion
While Halo’s efficacy for the use case of peaking is beyond debate, the question as to precisely why remains an active one. Hopefully as researchers wind through the process of experimentation and discovery in the task to solving urgent open questions about metabolic disease, we will benefit from that in ways that we might smartly use to further bodybuilding and physique applications.
We know that Halo uniquely antagonizes GR, imbuing it with effects that are synergistic with other AAS during caloric restriction (i.e., contest prep and cutting). We know that it is potently androgenic and can therefore supplant testosterone deep into prep that might otherwise cause bloating a la estradiol. And we know that it reduces fat mass and increases muscle mass. Well, if the shoe fits!
References
[1] Felici, F., Bazzucchi, I., Sgrò, P., Quinzi, F., Conti, A., Aversa, A., … Di Luigi, L. (2016). Acute severe male hypo-testosteronemia affects central motor command in humans. Journal of Electromyography and Kinesiology, 28, 184–192. doi:10.1016/j.jelekin.2015.12.004
[2] Dallek, R. & McGonagle, R. An unfinished life: John F. Kennedy, 1917-1963. Narrated by Richard McGonagle. Time Warner AudioBooks, New York, N.Y., 2003. Audiobook.
[3] Haggerty, D.F., Spector, E.B., Lynch, M., Kern, R., Frank, L.B., & Cederbaum, S.D. (1982). Regulation by Glucocorticoids of Arginase and Argininosuccinate Synthetase in Cultured Rat Hepatoma Cells. J Biol Chem 257, (5). 2246-2253.
[4] Vida, J. Androgens and anabolic agents. Academic Press, Inc. 1969.
[5] Ojasoo, T., Raynaud, J.-P., & Doré, J.-C. (1995). Correspondence factor analysis of steroid libraries. Steroids, 60(6), 458–469. doi:10.1016/0039-128x(95)00005-b
[6] Houtman, C. J., Sterk, S. S., van de Heijning, M. P. M., Brouwer, A., Stephany, R. W., van der Burg, B., & Sonneveld, E. (2009). Detection of anabolic androgenic steroid abuse in doping control using mammalian reporter gene bioassays. Analytica Chimica Acta, 637(1-2), 247–258. doi:10.1016/j.aca.2008.09.037
[7] Whorwood, C. B., Donovan, S. J., Flanagan, D., Phillips, D. I. W., & Byrne, C. D. (2002). Increased Glucocorticoid Receptor Expression in Human Skeletal Muscle Cells May Contribute to the Pathogenesis of the Metabolic Syndrome. Diabetes, 51(4), 1066–1075. doi:10.2337/diabetes.51.4.1066
[8] Fang, H., Tong, W., Branham, W. S., Moland, C. L., Dial, S. L., Hong, H., … Sheehan, D. M. (2003). Study of 202 Natural, Synthetic, and Environmental Chemicals for Binding to the Androgen Receptor. Chemical Research in Toxicology, 16(10), 1338–1358. doi:10.1021/tx030011g
[9] Becker, H., et al. “In vivo uptake and metabolism of 3H-testosterone and 3H-5α-dihydrotestosterone by human benign prostatic hypertrophy.” European Journal of Endocrinology 71.3 (1972): 589-599.
[10] Gupta, V., Bhasin, S., Guo, W., Singh, R., Miki, R., Chauhan, P., … Jasuja, R. (2008). Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Molecular and Cellular Endocrinology, 296(1-2), 32–40. doi:10.1016/j.mce.2008.08.019
[11] Furstenberger, C., Vuorinen, A., Da Cunha, T., Kratschmar, D. V., Saugy, M., Schuster, D., & Odermatt, A. (2012). The Anabolic Androgenic Steroid Fluoxymesterone Inhibits 11 -Hydroxysteroid Dehydrogenase 2-Dependent Glucocorticoid Inactivation. Toxicological Sciences, 126(2), 353–361. doi:10.1093/toxsci/kfs022
[12] Barbosa, J., Seal, U. S., & Doe, R. P. (1971). Effects of Anabolic Steroids on Hormone-Binding Proteins, Serum Cortisol and Serum Nonprotein-Bound Cortisol. The Journal of Clinical Endocrinology & Metabolism, 32(2), 232–240. doi:10.1210/jcem-32-2-232
[13] Akalestou, E., Genser, L., & Rutter, G.A. (2020). Glucocorticoid Metabolism in Obesity and Following Weight Loss. Frontiers in Endocrinology, 11. doi: 10.3389/fendo.2020.00059
[14] Spaanderman DCE, Nixon M, Buurstede JC, Sips HC, Schilperoort M, Kuipers EN, Backer EA, Kooijman S, Rensen PCN, Homer NZM, Walker BR, Meijer OC, Kroon J. Androgens modulate glucocorticoid receptor activity in adipose tissue and liver. J Endocrinol. 2018 Oct 1:JOE-18-0503.R1. doi: 10.1530/JOE-18-0503. Epub ahead of print. PMID: 30400038.
[15] Ruiz, D., Padmanabhan, V., & Sargis, P.M. (2020). Stress, sex, and sugar: Glucocorticoids and sex-steroid crosstalk to sex-specific misprogramming of metabolism. Endocrine Society. Aug 2020. 4(8). pp. 1 – 28. doi:10.1210/jendo/bvaa087
About the author
Type-IIx is a physique coach, author, and researcher. Bolus: A Practical Guide and Reference for recombinant Human Growth Hormone Use will be his first published textbook, anticipated for release in early 2023. Ampouletude.com will be Type-IIx's base of operations for coaching services and publications. Type-IIx is proud to be a contributing writer to MesoRx, his home forum, where he is a regular poster.
Leave a Reply
You must be logged in to post a comment.