
Sex hormones play a pivotal role in the development of gynecomastia: the benign enlargement of breast tissue in men. It is an esthetically unpleasant side effect which might result from anabolic steroid use. It is very common in the general population, too. Notably, gynecomastia develops in a lot of men during puberty. An endocrinology textbook notes that it initially occurs in 60 to 70 % of boys by 14 years of age (and then regresses within 1 to 2 years) [1]. In adults, the condition remains fairly prevalent as well. One study in healthy young men aged 18-26 years found gynecomastia in 40.5 % of them [2]. Another study came to very similar findings in healthy adult men aged 16-58 years, detecting palpable breast tissue in 36 % of them [3]. It should be pointed out that, in most cases (roughly 80 % of them), the diameter of the breast tissue did not exceed 4 cm. Thus, generally speaking, the gynecomastia in such cases is usually not extensively large.
Anabolic steroid usage and gynecomastia
So how common is gynecomastia as a result of anabolic steroid usage? Unfortunately, this is a difficult question to answer. An outpatient clinic for anabolic steroid users in the Netherlands notes that, among 160 of their patients, 34 % reported gynecomastia as a side effect [4]. However, it needs to be kept in mind that a certain bias exists when evaluating patients who are referred to such an outpatient clinic. It is inevitable that some degree of selection bias takes place—after all, those with little or no side effects from their anabolic steroid usage, are less likely to be referred to an outpatient clinic. Additionally, the side effect was self-reported. It’s been my experience that self-reported gynecomastia is pretty unreliable. Moreover, since it is well known that it is a side effect of anabolic steroid usage, AAS users are more aware of it and consequently are more likely to detect gynecomastia which was there prior to anabolic steroid usage. This might hold especially true in those who lose body fat during an anabolic steroid cycle, as the underlying tissue will become more palpable and gynecomastia might become more visible to the eye. Finally, I have also noticed a small number of users who—sometimes daily—palpate their breast tissue in quite an awkward way, actually pinching it. This can lead to inflammation of the underlying tissue which can definitely also feel, and look like, gynecomastia. This practice might also help to, at least partly, explain galactorrhea (breast discharge) that rarely appears to occur in some AAS users. ‘Breast self-manipulation’ is known to cause galactorrhea [5].
Let’s also have a look at some randomized-controlled trials that evaluated supraphysiological dosages of testosterone. Bhasin et al. randomized 21 men to receive 600 mg of testosterone enanthate weekly, and 19 to receive a placebo [6]. Two men receiving testosterone reported breast tenderness, but no gynecomastia was reported. Similarly, a later trial by Bhasin et al. randomized men to increasing dosages of testosterone enanthate: 50, 125, 300 or 600 mg weekly for 20 weeks, with or without the 5α-reductase inhibitor dutasteride [7]. Of the men receiving dutasteride, one of the twelve men in the 300 mg group reported nipple tenderness, and one of the fourteen men in the 600 mg reported nipple tenderness. Of the men not receiving dutasteride, one of the fifteen men in the 600 mg group reported nipple tenderness. In summary; 3 of 53 men receiving a supraphysiological dosage (300 or 600 mg weekly) of testosterone for 20 weeks reported nipple tenderness. Notably, again, no gynecomastia was reported. However, it does not appear that subjects were routinely evaluated by a physician to assess for gynecomastia. Development of small enlargement of breast tissue might therefore have gone unnoticed. In another trial, 271 subjects received 200 mg testosterone enanthate weekly, for a minimum of 6 months. Only 9 (3 %) of these subjects developed gynecomastia during treatment. Taken together, the risk of developing overt gynecomastia during the use of sole testosterone enanthate in dosages up to 600 mg weekly seems relatively small.
Unfortunately, no reliable data is present about dosages exceeding 600 mg weekly, nor about other compounds in supraphysiological dosages. However, given that estrogenic action lies at the root of causing gynecomastia (more about this later), we can make some cautious conclusions about higher dosages. An elegant trial from Bhasin’s group has demonstrated that the enzyme responsible for conversion of testosterone to estradiol, aromatase, starts to get saturated at dosages around 600 mg weekly [8]. This can be observed in the image below, where the dotted line represents the estimated estradiol concentration achieved given a certain total testosterone concentration.
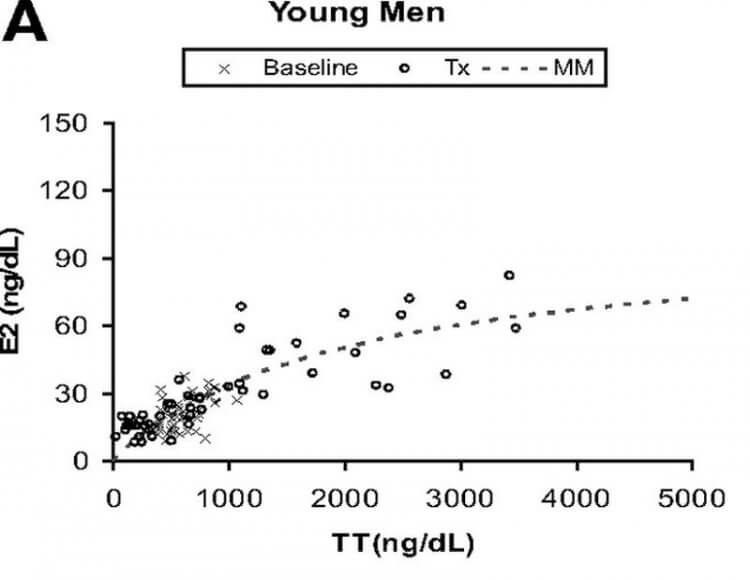
For context, a dosage of 600 mg weekly yields a nadir testosterone concentration of about 82 nmol/L (2,370 ng/dL) [9]. Given that the steady-state half-life of intramuscular testosterone enanthate injections is about 1 week [10], this means the testosterone concentration would’ve oscillated between roughly 82 and 164 nmol/L (2,370 and 4,740 ng/dL) over the course of a week. At these concentrations you can see that the corresponding estradiol concentrations are leveling off and aren’t rising that much anymore. Above roughly 150 nmol/L (4,320 ng/dL) there’s hardly any significant further increase in estradiol. The increase of testosterone leads to an increase of estradiol of proportionally lesser magnitude. As such, it can be derived that dosages above 600 mg of testosterone enanthate weekly aren’t that much more likely to cause gynecomastia, as there isn’t much of a further increase in estradiol anymore. (An exception to this are older men, in which saturation starts to take place at higher testosterone concentrations than in young men.)
Androgens and estrogens
The key players in the development of gynecomastia are androgens and estrogens. There’s consensus in the literature that it’s primarily caused by an imbalance between androgenic and estrogenic action on breast tissue [11]. In a nutshell: androgens inhibit its growth, whereas estrogens stimulate its growth. As such, a relative or absolute excess of estrogenic action, or a relative or absolute dearth of androgenic action on breast tissue is the culprit.
As is known, if you administer testosterone, you aren’t only increasing the androgens in your body. As described above, the enzyme aromatase will convert some of that testosterone into the estrogen estradiol. As a result, you’ll pretty much always see increased levels of estradiol in AAS users who administer high dosages of testosterone. However, it should be noted that this doesn’t decrease the androgen to estrogen ratio (which plays a part in gynecomastia development). This is especially true at higher dosages, as explained earlier because of saturation of the aromatase enzyme. This is perfectly illustrated in the picture below which I’ve taken from that same paper (keep in mind that it displays the estradiol to testosterone ratio rather than the inverse):
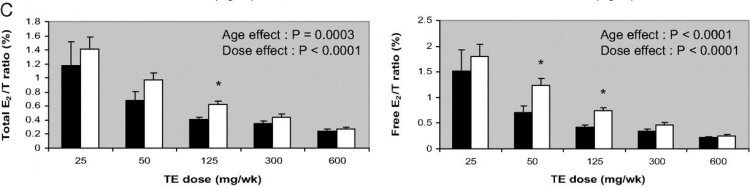
As a consequence, it seems unlikely that a diminished androgen to estrogen ratio is to blame for the development of gynecomastia on such a cycle. It seems more likely that the absolute excess of estrogen is the culprit. For completeness sake, however, the possibility remains that the ratio in serum is not reflective of what’s going on in the breast tissue itself. Unfortunately, no data is available about this.
Of course, the situation is vastly different right after a cycle. While the exogenous androgens clear from your body, your own endogenous production won’t be up and running immediately. There will be a transient period of time, at least several weeks to months, in which the endogenous production of testosterone is severely hampered. As a result, there is an absolute deficit of androgens in the body, similarly there’s also less estrogen. However, the androgen to estrogen ratio in this situation is often skewed towards the lower end. Meaning, there’s relatively more estrogenic action. This can lead to the development of gynecomastia post-cycle.
Prolactin
Prolactin is a hormone with lactogenic properties. This implies that it has the potential to stimulate milk production of the breasts. Moreover, it strongly suppresses the secretion of the gonadotropes LH and FSH [12], which, in turn, are responsible for testosterone production. As a result, the androgen to estrogen ratio can drop, which can lead to gynecomastia.
This happens in men who suffer from hyperprolactinemia, i.e., men with elevated levels of prolactin. This condition can be caused by tumors which secrete it (macroadenomas or prolactinomas). It can also occur in chronic renal failure (although this would lead to moderately elevated levels). Additionally, certain medications are known to elevate prolactin. Some notable ones are selective serotonin reuptake inhibitors (SSRIs), opiates and neuroleptics such as risperidone [13]. What’s of particular interest in the context of this article, is that estrogens and antiandrogens can also increase prolactin. In rare cases the conversion of testosterone to estradiol in testosterone replacement therapy has been noted to cause this [14]. Notably, androgens decrease prolactin [15]. As such, with exceedingly higher dosages of androgens, the nett effect will always head for suppression. If prolactin is increased during a cycle, it’s most likely the result of overt estrogenic action.
The direct role of prolactin in gynecomastia, if any, is unclear, to say the least. If it does play a role, it seems to be minor at best. For one, in a series of 30 gynecomastia cases, the majority (~80 %) of them didn’t appear to express the prolactin receptor in breast tissue [16]. If this holds true in general, then prolactin can only affect a small % of people their breast tissue. Besides that, there’s not really a clear mechanism through which prolactin induces gynecomastia. There’s just some speculation in the literature based on the effects of cross-regulation with growth hormone and progesterone in breast cancer cell lines [17]. In another study, researchers incubated two breast cancer cell lines with prolactin and this decreased androgen receptor mRNA and binding activity [18]. But if I’ve learned one thing from breast cancer cell lines, is that they behave awfully different from normal breast cells to external stimuli, especially hormones. (And just extrapolating something like this to the development of gynecomastia has little to do with science and is just an awful interpretation of cell studies.)
As a final note, I’ve often had people claiming to have high prolactin who, after performing bloodwork, turned out to have normal levels. You can’t “feel” your prolactin levels, you need to measure this to be sure.
Progesterone
Progesterone is one of those hormones which also takes a backstage role in male physiology. Just like androgens and estrogens, progesterone is a steroid hormone. Mostly, it’s important as a precursor to other steroid hormones (aldosterone and cortisol). It also affects the brain [19], including your sleep [20]. But I’m drifting off. The reason I’m mentioning progesterone in this article is because it’s purported to play a role in gynecomastia, and consequently, anabolic steroids.
First, AAS don’t increase progesterone, so there’s that. However, some anabolic steroids have some reasonable affinity for the progesterone receptor, and could thereby have progestogenic effects. And not only do they have affinity, a mammalian reporter gene bioassay even shows some activity for some AAS [21]. An excellent example would be mibolerone (aka Cheque Drops) and methyltrienolone, both seem to be quite potent at it. Other examples, to a lesser degree, would be trenbolone and nandrolone. Most other AAS, like testosterone, stanozolol, oxandrolone, methenolone, etc. don’t activate the progesterone receptor. Or, if they do, they only do it at absurd concentrations. (So in practice: they don’t.)
So then, could these compounds potentially lead to gynecomastia? Progesterone controls, to some extent, the proliferation and morphogenesis of the luminal epithelium of the breast (at least in women) [22]. What should be understood is that the luminal epithelium cells are the ones that shape the milk ducts. They don’t actually contribute to the breast mass itself, the lobular/glandular tissue. This tissue, from what I can find in the literature, is not affected by direct progesteronic action. What then remains, is an indirect role. Indeed, it’s been proposed that it amplifies the effect of estradiol on the breast tissue. Unfortunately, there’s no human data on this, there’s only a primate study which suggests this [23]. So any evidence linking progesterone to gynecomastia development is scarce at best. But what’s perhaps the best evidence against it, is a male contraceptive study in which men received 100 mg testosterone enanthate weekly in conjunction with a high dosage of the progestogen levonorgestrel (0.5 mg daily) for 6 months [24]. No gynecomastia was noted in any of the men.
Of course, now I’ve gotten you all curious about what’s the best way to treat gynecomastia. That’s gonna be food for a future article.
References
- Melmed, S., Auchus, R.J., Goldfine, A.B., Koenig, R.J., & Rosen, C.J. (2019). Williams Textbook of Endocrinology (14th ed.). Elsevier.
- Georgiadis, E., et al. “Incidence of gynaecomastia in 954 young males and its relationship to somatometric parameters.” Annals of human biology 21.6 (1994): 579-587.
- Nuttall, Frank Q. “Gynecomastia as a physical finding in normal men.” The Journal of Clinical Endocrinology & Metabolism 48.2 (1979): 338-340.
- Smit, Diederik L., and Willem de Ronde. “Outpatient clinic for users of anabolic androgenic steroids: an overview.” Neth J Med 76.4 (2018): 167.
- Rohn, Reuben D. “Benign galactorrhea/breast discharge in adolescent males probably due to breast self-manipulation.” Journal of Adolescent Health Care 5.3 (1984): 210-212.
- Bhasin, Shalender, et al. “The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men.” New England Journal of Medicine 335.1 (1996): 1-7.
- Bhasin, Shalender, et al. “Effect of testosterone supplementation with and without a dual 5α-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial.” Jama 307.9 (2012): 931-939.
- Lakshman, Kishore M., et al. “The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men.” The Journal of Clinical Endocrinology & Metabolism 95.8 (2010): 3955-3964.
- Bhasin, Shalender, et al. “Testosterone dose-response relationships in healthy young men.” American Journal of Physiology-Endocrinology And Metabolism (2001).
- Kaminetsky, Jed, Jonathan S. Jaffe, and Ronald S. Swerdloff. “Pharmacokinetic profile of subcutaneous testosterone enanthate delivered via a novel, prefilled single‐use autoinjector: a phase ii study.” Sexual medicine 3.4 (2015): 269-279.
- Narula, Harmeet S., and Harold E. Carlson. “Gynaecomastia—pathophysiology, diagnosis and treatment.” Nature Reviews Endocrinology 10.11 (2014): 684.
- De Rosa, Michele, et al. “Hyperprolactinemia in men.” Endocrine 20.1 (2003): 75-82.
- La Torre, Daria, and Alberto Falorni. “Pharmacological causes of hyperprolactinemia.” Therapeutics and Clinical Risk Management 3.5 (2007): 929.
- Sodi, R., et al. “Testosterone replacement-induced hyperprolactinaemia: case report and review of the literature.” Annals of clinical biochemistry 42.2 (2005): 153-159.
- Gooren, L. J. G., et al. “Prolactin secretion in the human male is increased by endogenous oestrogens and decreased by exogenous/endogenous androgens.” International journal of andrology 7.1 (1984): 53-60.
- Ferreira, M., et al. “Prolactin receptor expression in gynaecomastia and male breast carcinoma.” Histopathology 53.1 (2008): 56-61.
- Sansone, Andrea, et al. “Gynecomastia and hormones.” Endocrine 55.1 (2017): 37-44.
- Ormandy, Christopher J., et al. “Coexpression and cross-regulation of the prolactin receptor and sex steroid hormone receptors in breast cancer.” The Journal of Clinical Endocrinology & Metabolism 82.11 (1997): 3692-3699.
- Schumacher, Michael, et al. “Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors.” Progress in neurobiology 113 (2014): 6-39.
- Friess, Elisabeth, et al. “Progesterone-induced changes in sleep in male subjects.” American Journal of Physiology-Endocrinology and Metabolism 272.5 (1997): E885-E891.
- Houtman, Corine J., et al. “Detection of anabolic androgenic steroid abuse in doping control using mammalian reporter gene bioassays.” Analytica chimica acta 637.1-2 (2009): 247-258.
- Obr, Alison E., and Dean P. Edwards. “The biology of progesterone receptor in the normal mammary gland and in breast cancer.” Molecular and cellular endocrinology 357.1-2 (2012): 4-17.
- Zhou, Jian, et al. “Testosterone inhibits estrogen‐induced mammary epithelial proliferation and suppresses estrogen receptor expression.” The FASEB Journal 14.12 (2000): 1725-1730.
- Bebb, Richard A., et al. “Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach.” The Journal of Clinical Endocrinology & Metabolism 81.2 (1996): 757-762.
About the author
Peter Bond is a scientific author with publications on anabolic steroids, the regulation of an important molecular pathway of muscle growth (mTORC1), and the dietary supplement phosphatidic acid. He is the author of several books in Dutch and English, including Book on Steroids and Bond's Dietary Supplements.

Leave a Reply
You must be logged in to post a comment.