It is well-known that anabolic steroids can be damaging for the liver (hepatotoxic). In particular, oral anabolic steroids with a specific chemical alteration are the usual suspects for this. This is reflected in the blood by increases in aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT). Both of these can increase due to damage to the liver. Additional biochemical markers such as lactate dehydrogenase (LDH) and gamma-glutamyl transpeptidase (GGT) might also be increased, as well as serum bilirubin (especially when there’s more overt liver damage). Clinically, it can manifest itself in jaundice. Herein, the skin and the whites of the eyes turn yellowish as a result of an increase in serum bilirubin. Some rare cases of peliosis hepatis associated with anabolic steroid use have also been described in the literature [1, 2, 3]. In peliosis hepatis cystic blood-filled cavities pop up in the liver. Finally, some rare cases of hepatocellular carcinoma [4, 5, 6, 7] and adenoma [8, 9, 10] have also been reported in the literature.
A difficult question to answer is exactly how bad it is for the liver. Or perhaps more pragmatic: what are the chances of developing something serious, like jaundice, because of anabolic steroid use? There haven’t been a whole lot of clinical trials which have evaluated this in doses similar to that being used by bodybuilders. However, the trials that have done so, seemingly suggest that the risk is actually quite low. A follow-up question would be: what exactly causes these anabolic steroids to be hepatotoxic? What’s the mechanism behind it? And, consequently, is there anything someone could do to mitigate some of the damage? In this article I’ll attempt to answer these questions.
Markers of liver damage often increase, but risk of developing clinical signs of liver damage from anabolic steroid use is relatively small
Let’s have a look at some controlled trials with anabolic steroid use to see what they can tell us about the risk of developing clinical signs of liver damage.
In a double-blind randomized-controlled trial, subjects received either a placebo (n=28), oxymetholone (100 mg daily; n=30) or even more oxymetholone (150 mg daily n=31) [11]. These dosages are interesting since they’re right in the ballpark of dosages used by bodybuilders. Interestingly, they even received them for 16 weeks in a row. Additionally, it’s worth mentioning the subjects in this trial were HIV-positive. HIV-positive patients commonly already have their own array of health issues. Often coinciding with liver disease [12]. Bear this in mind when interpreting the results. An increase in biochemical markers of liver damage (ASAT, ALAT, GGT) was observed in 35 % of subjects in the 150 mg group and 27 % of subjects in the 100 mg group. Notably, total bilirubin did not change significantly in any study group. As such, no cholestasis developed in any of the subjects with the exception of one. One subject in the 100 mg group developed jaundice. This roughly puts the risk of developing jaundice (in HIV-positive patients) as a result of oxymetholone use in high dosages at 1 in 60.
In a similar vein, another double-blind trial randomized subjects to either 100 mg oxymetholone daily or a placebo for 24 weeks [13]. The subjects in this study were hemodialysis patients. Only 10% of patients demonstrated an increase in ASAT and ALAT. The increase was described as moderate and was transient in nature. Both of them showed no evidence of cholestasis. As such, no clinical signs of liver damage were found in this trial. A trial with the same dosage and duration, also in hemodialysis patients, was conducted by Supasyndh et al. [14]. The authors reported that none of the patients had an increase in ASAT more than three times the upper limit of normal, and 2 patients (9.5%) had an increase in ALAT more than three times the upper limit of normal. An increase in both values can be observed at the group level, with the means slightly exceeding the upper limit of normal. Additionally, a small increase in total and direct bilirubin was observed too (direct bilirubin is supposed to represent the conjugated fraction, although there are some caveats to the ‘direct’ measurement). These also are signs of liver damage. However, no clinical signs of liver dysfunction were reported.
A relatively large randomized, double-blind, placebo-controlled trial evaluated the effects of graded dosages of oxandrolone in HIV-infected men [15]. A total of 262 subjects were randomized to placebo, or to 20, 40 or 80 mg of oxandrolone daily. The study lasted for a total of 12 weeks. I’ll narrow in on the group receiving 80 mg daily (n=68). This is without a doubt a high dosage and representative of what can be seen in bodybuilders illicitly using anabolic steroids. Both ASAT and ALAT demonstrated a moderate increase. Total bilirubin values aren’t reported, but 1 subject in the 80 mg group (and 1 in the placebo group) had to discontinue due to WHO grade III or IV toxicity related to the total bilirubin value. Additionally, 9 had to discontinue because of this reason for the ASAT value, and another 9 for the ALT value. (These grades of toxicity boil down to at least 5-fold the upper limit of normal for ASAT and ALAT and at least 3 times the upper limit of normal of bilirubin.) Again, no clinical signs of liver damage were reported in the study.
There are more trials, but mostly in subjects receiving comparatively low doses of anabolic steroids that are not representative of its use in bodybuilders. These, indeed, also show no clinical signs of hepatotoxicity, but are simply not telling us much because of the low dosages.
A final remark I’d like to make in this section, is the interpretation of ASAT and ALAT values in the context of bodybuilders. While these enzymes are present in high concentrations in the liver, they’re also present in other tissues, such as muscle tissue. Both can increase as a result of muscular exercise too [16]. A nice addition, thus, would be the measurement of GGT. GGT can be found in the membranes of cells from various organs, including the kidney, pancreas, and of course the liver. In the liver, it’s found primarily in areas that are rich in biliary epithelial cells [17]. As such, GGT is increased in most diseases affecting the liver, gallbladder and bile ducts. An increase in GGT is a sensitive measure of cholestatic liver disease [18]. It can be viewed as a marker of cholestasis and obstruction of the bile ducts. However, again, it can also be increased by causes that are unrelated to the liver (as well as noncholestatic liver disease). Perhaps to get an even better view of liver damage one might incorporate a measurement of ALP too. The most pronounced elevations of ALP are seen in diseases associated with cholestasis, but smaller increases can be seen in all types of liver disorders [17]. The collective increase of ASAT, ALAT, GGT and ALP with concurrent anabolic steroid use should be interpreted as liver damage, and with a word of caution as obstruction of the bile ducts. (Measurement of bilirubin can provide insight in this.)
What causes AAS-induced hepatotoxicity? The oxidative stress hypothesis
This section is based on a paper William Llewellyn, Peter Van Mol (rest in peace), and I published in 2016 [19]. We believe the mechanism, in a nutshell, is roughly as follows. First an androgen binds to and activates the androgen receptor in liver cells. This androgenic activity leads to upregulation of carnitine palmitoyltransferase 1 (CPT1). This is the rate-limiting enzyme in fatty acid beta-oxidation. We believe this, in turn, leads to an increase in fatty acid beta-oxidation in the mitochondria of these liver cells. And, as a result, the production of reactive oxygen species (ROS) is increased. This increase then damages the liver cells, more specifically, its mitochondria.
We didn’t have a figure illustrating this hypothesis when we published our paper. However, I’ve had a figure drawn for my book, Book on Steroids in which this hypothesis is illustrated:
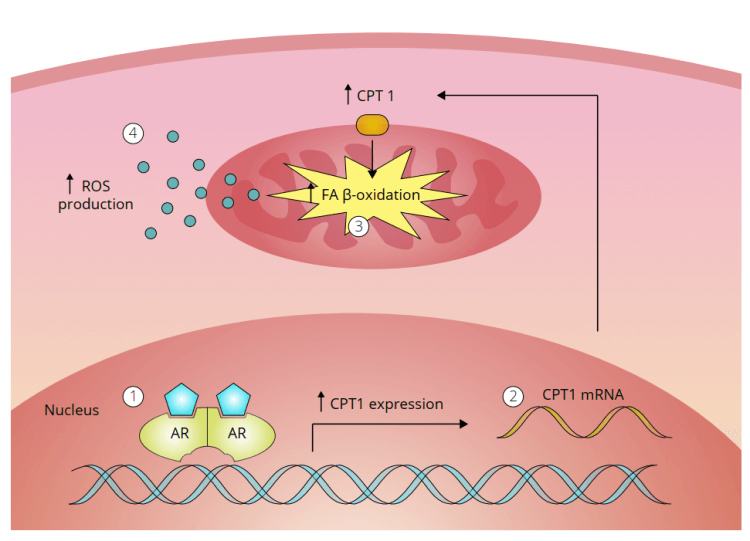
What this means is that androgenic action in liver cells leads to damage by ROS; oxidative stress. This implies that the hepatotoxicity of an anabolic steroid is dictated by two factors: its resistance to hepatic breakdown, and its potency to activate the androgen receptor. As such, anabolic steroids which are C-17α-alkylated, are hepatotoxic. Whereas those which aren’t, and thus are rapidly broken down in the liver, usually aren’t. Some exceptions to this might be cases in which metabolic breakdown of anabolic steroids in the liver is severely hampered. This might, for example, be the case with severe anemia. The enzymes responsible for oxidation of the C-17β-hydroxyl group are regulated by the redox status of the cell [20]. It doesn’t take too much imagination to figure out that severe anemia can impact the redox status of these cells. And it might do so to such an extent that it can retard the oxidation of the C-17β-hydroxyl group significantly. As a consequence, the cells might be exposed to higher concentrations of ‘regular’ anabolic steroids than what they would do under normal conditions. Thus conferring risk of hepatotoxicity.
Can anabolic steroid-induced hepatototoxicity be mitigated somehow?
If the above mechanism holds true, one could argue that mitochondrial antioxidants can mitigate the risk to some degree or another. In a rat study, silymarin (a mixture of various flavonoids extracted from silybum marianum, aka milk thistle), showed hepatoprotective effects in rats treated with methandienone (Dianabol) [21]. Indeed, there’s some evidence that it possesses antioxidant activity which might be responsible for its hepatoprotective effect [22]. However, that trial in rats was published in a rubbish journal, so I’d take it with a grain of salt.
Perhaps more interesting is the observational cohort study by Pagonis et al. [23]. Herein they observed 320 athletes; 160 were AAS users and the other 160 were not. A subset of these AAS users also used something called compound N. That is a substance that contains polyunsaturated phospholipids, including phosphatidylcholine, and vitamins of the B complex. Phosphatidylcholine is an important constituent of cell membranes (including those of the mitochondria) and plays an important role in bilirubin excretion into the bile. It’s a known antioxidant and the authors speculate that the B complex may potentiate the antioxidative effect of compound N.
In the trial, ASAT, ALAT, GGT and ALP increased in the AAS group not taking compound N compared to the AAS users who did take compound N and those who didn’t use AAS at all. The compound N group showed no increase in ASAT, ALAT, GGT and ALP compared to those not taking AAS. An important caveat, however, is that creatine kinase (CK) increased a lot more in the AAS users who didn’t take compound N compared to both other groups as well. Part of the increase in ASAT and ALAT in the AAS users who didn’t take compound N might therefore be explained by more muscle damage.
One can, of course, come up with additional (mitochondrial) antioxidants. Such as pyrroloquinoline quinone (PQQ), lycopene and vitamin E. However, there is simply no evidence to clearly indicate that these things can mitigate AAS-induced hepatotoxicity. The same goes for compound N in my opinion, as the trial was observational in nature rather than interventive. Moreover, it’s still to be seen how these reductions in surrogates of liver damage would translate to actual mitigation of clinical signs of liver damage. There’s simply very little clinical data about this.
References
- Nadell, J., and Jon Kosek. “Peliosis hepatis. Twelve cases associated with oral androgen therapy.” Archives of pathology & laboratory medicine 101.8 (1977): 405-410.
- Cabasso, A. L. A. N. “Peliosis hepatis in a young adult bodybuilder.” Medicine and science in sports and exercise 26.1 (1994): 2-4.
- Bagheri, Saeed A., and James L. Boyer. “Peliosis hepatis associated with androgenic-anabolic steroid therapy: a severe form of hepatic injury.” Annals of Internal Medicine 81.5 (1974): 610-618.
- Solbach, Philipp, et al. “Testosterone-receptor positive hepatocellular carcinoma in a 29-year old bodybuilder with a history of anabolic androgenic steroid abuse: a case report.” BMC gastroenterology 15.1 (2015): 1-7.
- Hardt, Aline, et al. “Development of hepatocellular carcinoma associated with anabolic androgenic steroid abuse in a young bodybuilder: a case report.” Case reports in pathology 2012 (2012).
- Kosaka, Atsushi, et al. “Hepatocellular carcinoma associated with anabolic steroid therapy: report of a case and review of the Japanese literature.” Journal of gastroenterology 31.3 (1996): 450-454.
- Boyd, Peter R., and Gene J. Mark. “Multiple hepatic adenomas and a hepatocellular carcinoma in a man on oral methyl testosterone for eleven years.” Cancer 40.4 (1977): 1765-1770.
- Smit, D. L., J. H. Nuijens, and W. de Ronde. “Spontaneous haemorrhage of hepatic adenoma in a patient addicted to anabolic steroids.” Netherlands Journal of Medicine 77 (2019): 261-263.
- Hernandez‐Nieto, Luis, et al. “Benign liver‐cell adenoma associated with long‐term administration of an androgenic‐anabolic steroid (methandienone).” Cancer 40.4 (1977): 1761-1764.
- Psatha, Evlampia A., et al. “Hepatocellular adenomas in men: MRI findings in four patients.” Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine 22.2 (2005): 258-264.
- Hengge, Ulrich R., et al. “Double-blind, randomized, placebo-controlled phase III trial of oxymetholone for the treatment of HIV wasting.” Aids 17.5 (2003): 699-710.
- Kaspar, Matthew B., and Richard K. Sterling. “Mechanisms of liver disease in patients infected with HIV.” BMJ open gastroenterology 4.1 (2017): e000166.
- Aramwit, P., et al. “Oxymetholone ameliorates insulin sensitivity in maintenance hemodialysis patients.” Clinical nephrology 71.4 (2009): 413-422.
- Supasyndh, Ouppatham, et al. “Effect of oral anabolic steroid on muscle strength and muscle growth in hemodialysis patients.” Clinical Journal of the American Society of Nephrology 8.2 (2013): 271-279.
- Grunfeld, Carl, et al. “Oxandrolone in the treatment of HIV-associated weight loss in men: a randomized, double-blind, placebo-controlled study.” JAIDS Journal of Acquired Immune Deficiency Syndromes 41.3 (2006): 304-314.
- Pettersson, Jonas, et al. “Muscular exercise can cause highly pathological liver function tests in healthy men.” British journal of clinical pharmacology 65.2 (2008): 253-259.
- Sotil, Eva U., and Donald M. Jensen. “Serum enzymes associated with cholestasis.” Clinics in liver disease 8.1 (2004): 41-54.
- Ruppin, David C., Michael I. Frydman, and Michael R. Lunzer. “Value of serum gamma‐glutamyltransferase activity in the diagnosis of hepatobiliary disease.” Medical Journal of Australia 1.10 (1982): 421-424.
- Bond, Peter, William Llewellyn, and Peter Van Mol. “Anabolic androgenic steroid-induced hepatotoxicity.” Medical Hypotheses 93 (2016): 150-153.
- Agarwal, Anil K., and Richard J. Auchus. “Minireview: cellular redox state regulates hydroxysteroid dehydrogenase activity and intracellular hormone potency.” Endocrinology 146.6 (2005): 2531-2538.
- Radovanović, Dragan, et al. “Hepatoprotective effects of silymarin in androgenic-anabolic steroid-induced liver damage.” Medicinski pregled 56 (2003): 79-83.
- Vargas-Mendoza, Nancy, et al. “Hepatoprotective effect of silymarin.” World journal of hepatology 6.3 (2014): 144.
- Pagonis, Thomas A., et al. “Multivitamins and phospholipids complex protects the hepatic cells from androgenic-anabolic-steroids-induced toxicity.” Clinical Toxicology 46.1 (2008): 57-66.
Main image by zachvanstone8 from Pixabay
About the author
Peter Bond is a scientific author with publications on anabolic steroids, the regulation of an important molecular pathway of muscle growth (mTORC1), and the dietary supplement phosphatidic acid. He is the author of several books in Dutch and English, including Book on Steroids and Bond's Dietary Supplements.

Leave a Reply
You must be logged in to post a comment.