
It was reported on the Internet that 34-year old IFBB Pro Bodybuilder Kris Dim had a near fatal stroke or aortic dissection this month while training. He also underwent emergency heart surgery which was successful. Of course, many people are going to speculate about the causal role of steroids in strokes and heart disease and pathology. Do you think there is any relationship between resistance training and/or AAS and this medical condition?
Should recreational and competitive bodybuilders who use steroids be concerned? What type of testing/procedures can thoroughly determine current “heart health”?
This is a wide-open question and it is not possible to answer these questions adequately and still maintain interest and clarity. For this reason the response will be limited to those areas of possible relevance to medical concerns surrounding Kris Dim. The question includes information that is inconsistent with that found online but includes the important points. The information considered for the discussion below is that Kris Dim collapsed while training on June 9 and had emergency heart surgery to repair a split in his aortic valve. The surgery cited is for an acute aortic dissection (AAD) and replacement of a split aortic valve. Kris Dim also suffered an apparent stroke which resolved. There are some sites reporting that he also suffered a heart attack.
There is little doubt that Kris Dim has a very serious medical condition. As with other acute medical events the upcoming days and weeks are critical in this young man’s life. An important part of recovery is the support and wishes of others. I would encourage individuals to send encouraging messages and good will for his recovery and rehabilitation.
Kris Dim
2300 O Street
Sacramento, CA 96816
The discussion will be in two parts. Part I is the association between anabolic-androgenic steroids (AAS) and AAD. Comments will be added on a general nature between AAS and cardiovascular disease. Part II is the pathophysiology of AAD.
Part I. Anabolic-Androgenic Steroids and Acute Aortic Dissection.
There is no association, link, or otherwise known between AAS and AAD. Many are quick to suggest a link between AAS and a medical condition simply by their existence in an individual. With over one million illicit AAS users matched by an equal number of licit users the link or association would be easily discoverable. This, however, does not stop the hysteria to suggest, wildly, for a connection. What is the evidence for any connection or association between AAS and AAD? Or, as the literature has possibly suggested weight lifting and thereby indirectly AAS.
In 2007, a retrospective study from the Section of Cardiothoracic Surgery, Yale University School of Medicine reported on their findings for evidence between weight lifting and aortic dissection.[1]They collected 31 cases (19-76 years of age) of acute aortic dissection “in the context of severe physical exertion where weight lifting (of various types) occurred.” The methodology used was, “Cases were culled from retrospective review of a large university data base and from reports forwarded to our attention from around the country.” The mortality rate overall was ~33% and of those receiving surgical intervention, 17%. It is also stated that, “Moderate aortic dilatation confers vulnerability to exertion-related aortic dissection.” The authors suggest, “Routine echocardiographic screening of individuals engaging in heavy strength training should be considered, in order to prevent this tragic loss of life.”
The authors mention a prior report of theirs from 2003. Rather than a report this is a letter to the editor.[2]In the letter they describe five cases (19-53 years of age) of “aortic dissection of the ascending aorta in the setting of high-intensity weight training or other strenuous exercise.” The physical exertion is further detailed as, “Two patients were weight training, a third was attempting to move a heavy granite structure, and the other 2 were doing push-ups.”
Previous reports cite weight lifting and aortic dissection. In 1990, there is a report of four cases.[3]Two of the four have hypertension and two were exercising when symptoms developed. All four had aortic medial degeneration. From 1993 to 2005, single case reports of weight lifting and aortic dissection are published. These describe a descending aortic dissection,[4]underlying aortic pathology of myofibroblastic proliferation of the aortic adventitia consistent with nodular fasciitis,[5]a 28-year-old previously healthy male who presented to the ER complaining of severe anterior chest pain beginning during a workout found to have an ascending aortic dissection with cystic medial degeneration,[6]and a case of a symptomatic young weightlifter who died from an aortic dissection, and upon autopsy, was diagnosed as having non-Marfan’s fibrillinopathy.[7]
These reports are not evidence for any link or association (for anything!). AAS use is mentioned for two of the above patients. The weight lifting when taken in context is anything from progressive resistance training (classical weight lifting) to push-ups. There is even a plausible suggestion that the increased intrathoracic pressure generated while weight lifting (i.e., straining) will cause an uplifting or tear in the artery wall leading to the dissection. Of course, this feeds into those willing to connect AAS to Hurricane Katrina (more on that link at a later time). These studies did what they set out to do — find something, in this case weight lifting. In every study to date that reviews AAD none find any association or risk with weight lifting.
Moreover, the recommendation by the authors for a, “Routine echocardiographic screening of individuals engaging in heavy strength training should be considered, in order to prevent this tragic loss of life,” is absurd. This comment has undoubtedly caused more harm and worry than any good that was intended by their ridiculous suggestion. It is not worth an analysis but since the comments have reached the public I feel one is necessary, albeit brief.
First, as stated above there is no association, none. They may have well as just said that all individuals who sneeze also obtain this procedure. Multiple studies have shown AAD in patients with no predisposing factors and even in one that sneezed. As early as 1981 aortic dissection was reported in a 33-year-old man with no predisposing factors but found to have pathology of cystic medial degeneration.[8]Aortic dissection in a healthy 32-year-old[9]and healthy 14-year-old[10]have been reported with no predisposing factors. Finally, in 2005, acute aortic dissection provoked by a sneeze is reported.[11]
Second, the variable that these authors suggest as a determinant factor in AAD is aortic dilatation. This has not been shown to be a determining factor in AAD. Nevertheless, what measure by what technology is to be measured. The techniques and instrumentation for echocardiography are numerous.
Third, even if one were to assume that screening would identify susceptible individuals what would be the recommended treatment. Do not sneeze. Do not do push-ups. Do not breathe!
Fourth, what is the expected number of AAD cases for the general population? If weight lifting increases the risk for AAD, it would than be expected to see more cases than that for the general population. For example, if there were 1,000 (one thousand) weight lifters in a country with a population of 100,000 and the incidence was 1 case per 1,000 people per year and there was a finding of 2 cases or more in the weight lifters this would be of significance and importance.
The incidence of aortic dissection ranges from 5 to 30 cases per million people per year.[12]Assuming a U.S. population of 250 million people, this an expected case rate of 1250 to 7500 cases per year. This is similar to the average reported elsewhere for roughly2000 new cases per year in the United States. However, we are interested only in those that lift weights. This population is more than one million but for argument this number can be used (note: illicit AAS use are numbers around one million). Thus, in weight lifters based on general population studies one expects to see 5 to 30 cases per year. From the above studies, the total number of cases reported for more than five years does not total 40 or even 50. The reported cases is within that expected from general population estimates, and much less than the higher estimate of 30 cases per year.
There are more criticisms of this report but, personally, this is enough of a waste of time. A more important association and one that is well-known to most is the risk factor of a bicuspid aortic valve. Schwarzenegger underwent surgery for a bicuspid aortic valve years ago. This is a known risk factor for heart disease. The mention of a “split aortic valve” for Kris Dim makes this a strong possibility (once again, this is only conjecture and speculation).
Part II. Acute Aortic Dissection
An excellent review of AAD can be foundonline.[13]A discussion of AAD must include some knowledge of anatomy. Below is a brief anatomy primer by the use of images followed by AAD pathophysiology.
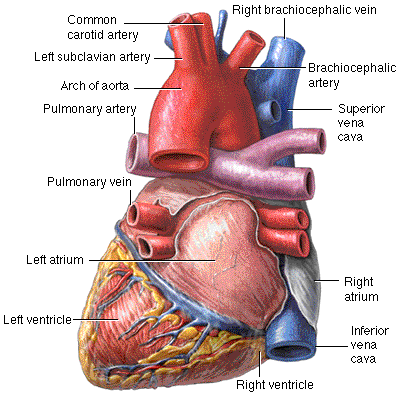
The relationship of the heart and great vessels. The branches of the aortic arch are identified.
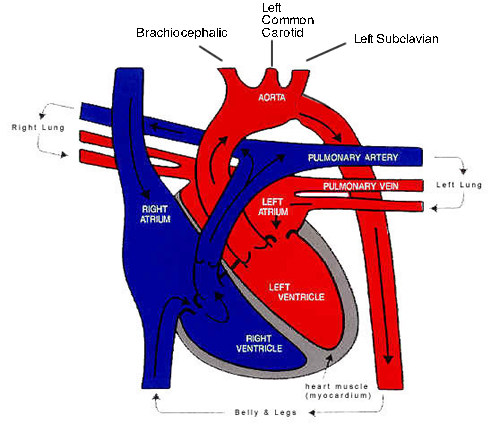
A schematic representation of the heart and great vessels. This clearly shows the relationships of the great vessels and aortic arch branches.

The aortic arch and the major branches. Note the right common carotid and right subclavian are branches of the brachiocephalic while the left common carotid and left subclavian form individual branches off the aorta.
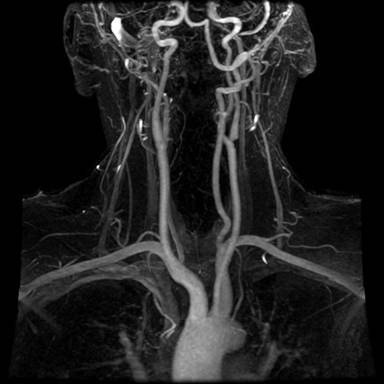
A radiographic imaging of the aortic arch and associated vessels. One can superimpose the above vessel diagram for identifications.
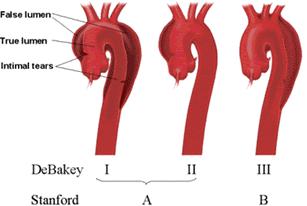
The adventitia provides most of the tensile strength of theaortic wall with little contribution from the media. The mediais composed of concentrically arranged smooth muscle interposedwith connective tissue proteins such as collagen, elastin, andfibrillin within the ground substance. Aorta dissection occurs as blood flow is redirectedfrom the aorta (true lumen) through an intimal tear into themedia of the aortic wall (false lumen). A dissection plane thatseparates the intima from the overlying adventitia along a variablelength of the aorta is created within the media. Classification of aortic dissections. Stanford classification: Type A dissections involve the ascending aorta independent of site of tear and distal extension; type B dissections involve transverse and/or descending aorta without involvement of the ascending aorta. DeBakey classification: Type I dissection involves ascending to descending aorta; type II dissection is limited to ascending or transverse aorta, without descending aorta; type III dissection involves descending aorta only.
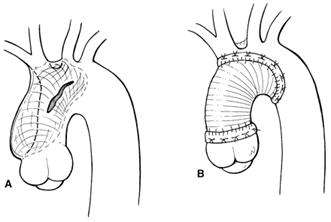
A typical AAD repair. The primarytear is usually greater than 50% of the circumference of theaorta, but the full circumference is rarely involved. The primarytear in type A dissection is usually located on the right anterioraspect of the ascending aorta and follows a somewhat predictablecourse, spiraling around the arch and into the descending thoracicand abdominal aorta on the left and posteriorly.
The incidence of aortic dissection ranges from 5 to 30 cases per million people per year, depending on the prevalence of risk factors in the study population.[14]In the United States, this results in roughly2000 new cases per year. The prevalence range estimates from 0.2 to 0.8 per100,000 per year (2 to 8 per 106per year) [300,000,000/100,000 = 3,000; 3,000 X (0.2-0.8) = 600-2400/year]
Although the disease is uncommon, its outcome is frequently fatal, and many patients with aortic dissection die before presentation to the hospital or prior to diagnosis. The clinical outcome is eventuallydetermined by dissection type and timing of presentation, patient-relatedfactors, and the quality and experience of the individuals andinstitution providing care.
The most common site of initiation of aortic dissectionis the ascending aorta (50%).Fifty percent of patients suffering acute type A aortic dissectionare dead within 48 hours. Acute type A dissection is complicated by aortic valve insufficiencyin up to 75% of patients.
Surviving the operation for acute dissection represents thebeginning of a lifelong requirement for meticulous medical managementand continued close observation. The published results for long-term survival following acutetype A dissection surgically treated over the last decade isroughly 55% to 75% at 5 years and between 32% and 65% at 10years.
Aortic dissection should always be considered in the settingof severe, unrelenting chest pain, which is present in mostpatients. Patients usually have no previous episodes of similarpain and are often quite anxious. The character of the pain is often describedas “ripping” or “tearing” and is constant with greatest intensityat the onset. Patients may also have signs or symptoms related to malperfusionof the brain, limbs, or visceral organs.
While pain is the most common symptom of aortic dissection, more than one-third of patients may develop a myriad of symptoms secondary to the involvement of the organ systems. The symptoms of aortic dissection may mimic myocardialischemia. Myocardial ischemia and rupture intothe pericardium are the cause of death in as many as 80% ofdeaths from acute dissection. Regardlessof whether the true and false lumen communicate, perfusion ofaortic side branches may be compromised by the dissection causingend-organ ischemia. Involvement of the brachiocephalic vessels withloss of brain perfusion may result in transient syncope or stroke.Strokeis a presenting feature in fewer than 5% of patients with acutetype A dissection. Loss of perfusion to intercostal or lumbararteries may result in spinal cord ischemia and paraplegia.
There are several hypotheses regarding the etiology of the intimaldisruption (primary tear) that permits aortic blood flow tocreate a cleavage plane within the media of the aortic wall. These include cystic medial necrosis or degeneration, intramural hematoma, and penetrating atherosclerotic ulcers.
Several risk factors have been identified that can damage theaortic wall and lead to dissection. Hypertension is themechanical force most often associated with dissection and isfound in greater than 75% of cases. Other suggested risk factors include connective tissue disorders (Ehlers-Danlos syndrome, Marfan disease, Turner’s syndrome), cystic medial disease of aorta, aortitis, iatrogenic, atherosclerosis, thoracic aortic aneurysm, bicuspid aortic valve, trauma, pharmacologic, coarctation of aorta, hypervolemia (pregnancy), congenital aortic stenosis, polycystic kidney disease, pheochromocytoma, Sheehan’s syndrome, Cushing’s syndrome.
The bicuspid aortic valve (BAV) is the most common congenitalcardiac malformation, occurring in 1% to 2% of the population.The majority of BAV patients develop complications requiringtreatment. Serious complications will develop in>33% of patientswith BAV. Although patients with BAV may go undetectedor without clinical consequences for a lifetime, the vast majoritywill require some intervention, most often surgery. The importantclinical consequences of BAV disease include aortic dissection.[15]
A bicuspid aortic valve is present in 1%to 13% of unselected cases of aortic dissection. In threeother large series, the figure was approximately 7%, but in15% of proximal dissections. The presence of a bicuspid aorticvalve increases the risk of dissection nine-fold (6.14%v0.67%),and this rises to 18-fold if there is a unicommissural aorticvalve (12.5%v0.67%). Aortic dissection occurs at a youngerage in patients with a bicuspid aortic valve. Twenty four per cent of a group of patientswho died from aortic dissection before the age of 40 had a bicuspidaortic valve, and 13% of (young) military personnel. Mostpatients with aortic dissection have hypertension. Aortic dissection usually occursin the presence of a normally functioning bicuspid aortic valve but it may also occur with stenosed bicuspid aortic valves. Aortic root dilatation, a precursorof dissection, occurs in 50-60% of patients with a normally functioningbicuspid aortic valve and has been reported as often withnormally functioning bicuspid aortic valves as in patients withassociated mild aortic regurgitation or mild to moderate aorticstenosis.[16]

References
[1]I. Hatzaras, M. Tranquilli, et al.,Weight lifting and aortic dissection: more evidence for a connection, 107(2) Cardiology 103 (2007).
[2]J.A. Elefteriades, I. Hatzaras, et al.,Weight Lifting and Rupture of Silent Aortic Aneurysms, 290(21) JAMA 2803 (2003).
[3]C. de Virgilio, R.J. Nelson, et al.,Ascending aortic dissection in weight lifters with cystic medial degeneration, 49(4) Ann Thorac Surg 638 (1990)
[4]J.S. Schor, M.D. Horowitz, et al.,Recreational weight lifting and aortic dissection: case report, 17(4) J Vasc Surg 774 (1993)
[5]D.N. Gwan-Nulla, W.R. Davidson, Jr., et al.,Aortic dissection in a weight lifter with nodular fasciitis of the aorta, 69(6) Ann Thorac Surg 1931 (2000)
[6]M.V. Ragucci and H.G. Thistle,Weight lifting and type II aortic dissection. A case report, 44(4) J Sports Med Phys Fitness 424 (2004)
[7]C.J. Hogan,An aortic dissection in a young weightlifter with non-Marfan fibrillinopathy, 22(4) Emerg Med J 304 (2005)
[8]C.B. Loeppky, M.A. Alpert, et al.,Extensive aortic dissection from combined-type cystic medial necrosis in a young man without predisposing factors, 79(1) Chest 116 (1981)
[9]T. Bey and K. Sturmann,Aortic dissection in a healthy 32-year-old man without risk factors, 2(1) Eur J Emerg Med 56 (1995)
[10]R. Jeganathan, B. Badmanaban, et al.,Spontaneous aortic dissection in a 14-year-old adolescent, 20(5) J Card Surg 490 (2005)
[11]A. Baydin, M.S. Nural, et al.,Acute aortic dissection provoked by sneeze: a case report, 22(10) Emerg Med J 756 (2005)
[12]I.A. Khan and C.K. Nair,Clinical, Diagnostic, and Management Perspectives of Aortic Dissection, 122(1) Chest 311 (2002).
[13]Green GR, Kron IL.Aortic Dissection. In: Cohn LH, Edmunds LH Jr, eds. Cardiac Surgery in the Adult. New York: McGraw-Hill, 2003. Available at: http://cardiacsurgery.ctsnetbooks.org/cgi/content/full/2/2003/1095#.
[14]I.A. Khan and C.K. Nair,Clinical, Diagnostic, and Management Perspectives of Aortic Dissection, 122(1) Chest 311 (2002).
[15]P.W.M. Fedak, S. Verma, et al.,Clinical and Pathophysiological Implications of a Bicuspid Aortic Valve, 106(8) Circulation 900 (2002)
[16]C. Ward,Clinical significance of the bicuspid aortic valve, 83(1) Heart 81 (2000).
About the author
The research of Michael Scally focuses on returning individuals to normal physiology after the discontinuation of anabolic steroids. Dr. Scally has presented his medical protocol for the treatment of Anabolic Steroid Induced Hypogonadism before the Endocrine Society, American Association of Clinical Endocrinologists, American College of Sports Medicine, and International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV. Dr. Scally is the author of "Anabolic Steroids - A Question of Muscle: Human Subject Abuses in Anabolic Steroid Research."
Leave a Reply
You must be logged in to post a comment.