In my previous article I discussed how there’s an increased mortality and morbidity risk when systolic blood pressure increases beyond 115 mmHg and diastolic blood pressure exceeds 75 mmHg [1]. A rough indication in how much anabolic steroids tend to affect blood pressure is an increase of around 5 to 10 mmHg in systolic blood pressure, and half of that for diastolic blood pressure. Concurrent use of other (performance-enhancing) drugs could further affect this. Having an increased blood pressure for a transient period of time isn’t particularly so problematic, however, a lot of anabolic steroid users tend to use anabolic steroids for years. Then it’s a different story.
In this article I will lay out how you can monitor your blood pressure, when it’s wise to start treating it, and how you can potentially treat it.
How to measure your blood pressure
First, of course, you need a device to measure your blood pressure at home. I would express my strong recommendation to do this with an automated electronic device that measures it at the level of the upper arm. These are reliable and require the least skill and therefore there’s the least chance you’re doing something wrong when measuring it. They are readily available and cost somewhere around $50. They’re well worth the investment. Most of my clients use devices from Omron, but I’m sure there are plenty of other different brands out there which make excellent devices too.
Additionally, make sure that the blood pressure-measuring device comes with a cuff size that is appropriate for the size of your arm. Usually, blood pressure-measuring devices come with a cuff size M, which is meant for upper arms with a circumference of up to 31—33 cm. Of course, this isn’t the arm circumference for your flexed biceps, but the circumference when having your arm slightly bent without flexing. Most AAS users have arms larger than that. In most cases a cuff size L is appropriate, which fits upper arms with a circumference of up to 41—43 cm. If you’re a very big fella, you might need size XL, which fits upper arms with a circumference of up to around 51—53 cm.
An appropriate cuff size is important because if it’s too small, it might overestimate your blood pressure. This is nicely illustrated in a trial which examined the differences in blood pressure between a cuff size M and a cuff size L in 193 bodybuilders competing at the Mexican National Bodybuilding and Fitness Championship [2]. Those whom had arms which were too big for cuff size M (>33 cm) had a 8.2 mmHg higher systolic blood pressure with this cuff compared to cuff size L. Diastolic blood pressure was 1.6 mmHg higher.
A different study, in obese individuals, also underscored how important appropriate cuff size is [3]. For every 5 cm increase in arm circumference beyond 35 cm, there was a 2—5 mmHg increase in systolic blood pressure and a 1—3 mmHg increase in diastolic blood pressure.
Now you’re ready to measure it, how should you do it? The International Society of Hypertension has a great picture which illustrates this [4], let’s have a look:
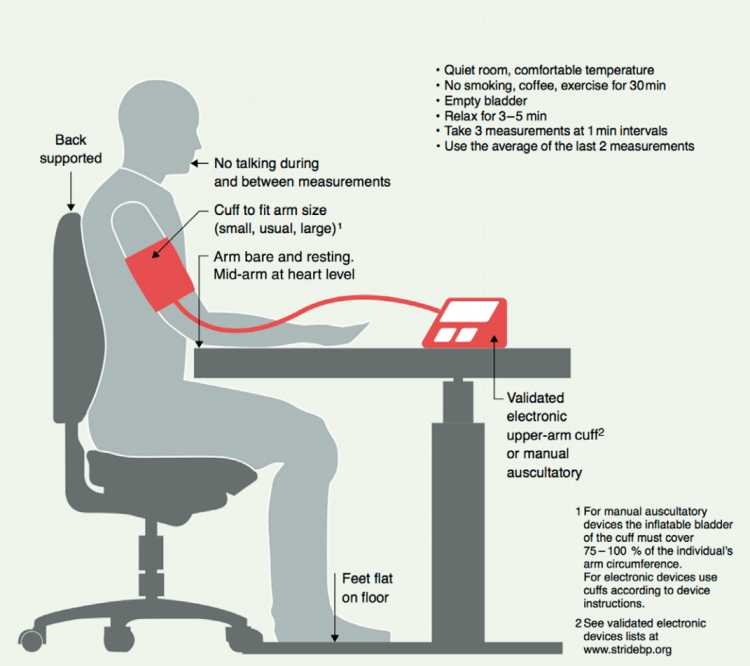
Make sure you don’t have to pee, don’t smoke (of course, you don’t, right?), drink coffee/caffeine or exercise for 30 minutes prior to measuring, and get your ass into a quiet comfortable room where you chill for a couple of minutes. You’re seated on a chair that properly supports your back behind a desk. You put the cuff on and you rest your arm on the desk, completely resting it with mid-arm at heart level. You put your feet on the floor, don’t cross your legs, and chill as hard as you can and get the device to measure your blood pressure. You repeat the measurement twice with a little pause between them and voila. You take the average of the last 2 measurements and write them down somewhere (most electronic blood pressure devices have a memory function too, so you can skip the writing).
So how often should you do these measurements? The European Society of Hypertension Practice Guidelines for home blood pressure monitoring recommend to, initially, do this at least 3 and preferably 7 days before you consider treating your blood pressure [5]. The measurements should be taken both in the morning and the evening. After this initial period, measuring it about once a week suffices.
When to start treating your blood pressure
After reading my previous article and the first paragraph of this one, you might think you should treat your blood pressure once it’s higher than 115/75 mmHg. However, a recent systematic review and meta-analysis found that, in primary prevention, lowering blood pressure only reduces mortality and cardiovascular disease risk if baseline systolic blood pressure is 140 mmHg or higher [6]. If it was lower than that at baseline, the authors were unable to find any benefit in regard to mortality or cardiovascular disease risk. Indeed, this is also the reason why the European Society of Hypertension classifies hypertension as an office systolic blood pressure equal or higher than 140 mmHg and/or diastolic blood pressure equal or higher than 90 mmHg [5]. They define hypertension as the level of blood pressure at which the benefits of treatment (either with lifestyle intervention or drugs) unequivocally outweigh the risks for treatment, as documented by clinical trials.
It should be emphasized that this 140/90 mmHg threshold concerns office blood pressure measurements. These are usually slightly higher compared to blood pressure measurements done at home. As such, the threshold for home blood pressure measurements is defined as a mean value of 135/85 mmHg [5].
The treatment of hypertension yields clear clinical benefits. A meta-analysis shows that every 10 mmHg decrease in systolic blood pressure reduces the risk of major cardiovascular events by 20 %, of coronary heart disease by 17 %, of stroke by 27 %, of heart failure by 28 %, and of all-cause mortality by 13 % [7]. Unfortunately, treating hypertension doesn’t fully negate all the risks that are seen in large observational studies. Two likely reasons for this are: 1) being hypertensive for prolonged periods of time can irreversibly damage certain organs, treatment won’t undo the damage done, and 2) it often occurs in conjunction with several other comorbidities which can affect outcome (e.g. obesity). The first reason can be covered by commencing treatment as soon as possible when required, and the second is not particularly applicable to a drug-induced increase in blood pressure (as with AAS). However, it should definitely not be forgotten that, outside of the modest increase in blood pressure AAS cause, they negatively impact health. So fixing your blood pressure, of course, doesn’t fully negate the health risks of AAS—much like how it doesn’t fix the health risks of other comorbidities which often go in parallel with hypertension.
Treating high blood pressure: lifestyle changes
Just as in the general population, there might be certain lifestyle changes you could adopt to decrease your blood pressure before you reach out to drugs to lower your blood pressure. However, in some cases drugs are to be used immediately as well in conjunction with lifestyle changes. The European Society of Hypertension recommends to immediately start drug treatment in those with a high or very high risk of cardiovascular disease, renal disease or hypertension-mediated organ damage. It also recommends immediate drug treatment in all patients who have a blood pressure equal or higher than 160/100 mmHg. In these cases, I would urge you to stay clear of anabolic steroid usage and reach out to your physician to get treated. I would strongly discourage anabolic steroid use if this applies to you.
Having said that, here are some lifestyle changes. An obvious one being to quit smoking if you do. Not so much for lowering blood pressure, but simply because smoking vastly increases the risk of cardiovascular disease. But reading this probably won’t make you quit smoking if you do (if it only were that easy right?), just wanted to put it out there.
An effective strategy is to reduce dietary sodium, i.e. salt. Several lines of evidence have consistently implicated dietary salt intake with cardiovascular risk [8]. A 2013 Cochrane meta-analysis of 34 randomized-controlled trials demonstrated a decrease in blood pressure of 4.2/2.1 mmHg for every 4.4 g/day reduction in salt intake (=1.8 g of sodium) [9]. Consequently, the European Society of Hypertension recommends salt intake to be limited to 5 g daily (= 2 g sodium) [5]. However, dietary salt intake shows a U-curve with regard to risk of cardiovascular events and death. Meaning, while lowering salt intake will decrease this risk, it starts to increase again below a certain daily intake. A recent meta-analysis found that, compared to a sodium intake of 7 g or more daily, 4–5 g daily had a lower risk of cardiovascular events and death [10]. Similarly, an intake of less than 3 g daily also showed an increased risk compared to a sodium intake of 4–5 g daily. (The studies actually looked at urinary sodium excretion as a proxy for sodium intake. This is excellent as a proxy, so in this article I pretend they are the same.) It is not completely clear what causes this, since 3 g sodium daily is plenty to cover daily requirements. The European Society of Hypertension clings on the blood pressure-lowering effect as being decisive to further reduce it anyway. Feel free to do so, but I guess it might be more pragmatic to stick to the 4–5 g daily unless you already have a lower intake, then leave it at that. And then realize a further blood pressure reduction, if required, with further lifestyle intervention or drugs. Finally, something I can’t stress enough: check everything you eat for their salt contents. You might be surprised at the salt contents of some of the products you consume.
If you drink a lot of alcohol, it’s of course also wise to moderate that (or abstain from drinking it). The blood pressure-lowering effect of this is very modest (~1.2/0.7 mmHg decrease [11]), but alcohol is shit for you (cardiovascular) health regardless. The recommendation is to limit consumption to 14 units per week for men (8 per week for women; 1 unit is equal to 125 mL of wine or 250 mL of beer) [5]. And, other than that, add in some aerobic exercise to your routine if you haven’t and just make sure you don’t get fat. That helps too.
Treating high blood pressure: drugs
Several drugs are available for the treatment of hypertension. There are five major classes of drugs that are recommended for this: ACE inhibitors, angiotensin receptor blockers (ARBs), beta-blockers, calcium channel blockers (CCBs), and thiazide(-like) diuretics. There are some small differences with regard to cause-specific differences on outcomes between these drugs. However, overall major cardiovascular outcomes and mortality are similar, and as such all are recommended by the European Society of Hypertension as first-line treatment [5]. The International Society of Hypertension also recommends these drugs as first-line treatment, with the exception of beta-blockers [4]. Each class of drugs has its own contraindications. For example, athletes and physically active patients are noted as a possible contraindication for the use of beta-blockers, and a heart rate below 60 bpm is noted as a compelling contraindication for both beta-blockers and certain calcium channel blockers (the nondihydropyridines) [5]. Beta-blockers are generally added to treatment when there is a specific indication for their use. Additionally, thiazide-like diuretics are prefered over thiazides. As such, it boils down to the dihydropyridine calcium channel blockers, ACE inhibitors, ARBs and the thiazide-like diuretics. It’s been my experience that AAS users have relatively easier access to the latter three, and not so much the calcium channel blockers. (Of course, unless they get it prescribed by their physician, but then you just do whatever he prescribes you.) As such, I will focus on these three treatment modalities.
Both ACE inhibitors and ARBs hook into the so-called renin-angiotensin-aldosterone system (RAAS). This is a hormonal system that plays an extremely important role in the regulation of blood volume, electrolytes and systemic vascular resistance. As such, it forms a very interesting target for the treatment of hypertension. This hormonal system works as follows. The kidneys release an enzyme called renin whenever it detects a drop in blood pressure. This enzyme, in turn, converts a protein that is produced by the lever, angiotensinogen, into angiotensin I. This is a small peptide that forms the substrate for angiotensin-converting enzyme (ACE), which cuts off an additional two amino acids from this peptide yielding angiotensin II. Angiotensin II is responsible for vasoconstriction, predominantly in the arterioles. Consequently, this increases blood pressure—and that closes the loop that was started by the detection of a drop in blood pressure by the kidneys. Additionally, angiotensin II inhibits the process of excretion of water (diuresis) and sodium (natriuresis) by the kidneys. It achieves this effect in part by evoking release of aldosterone by the adrenals. Aldosterone is a ligand for the mineralocorticoid receptors (MR) that are located in the kidneys. MR activation results in water and sodium retention and potassium secretion. RAAS is schematically pictured below:
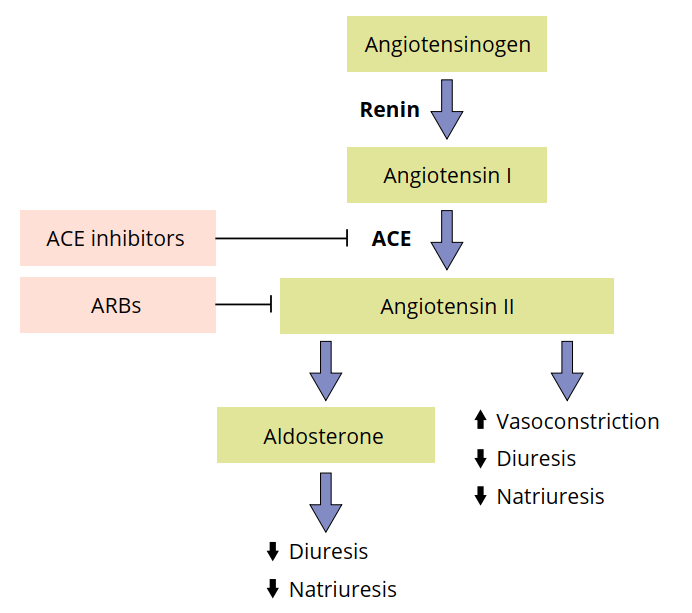
Now you know how RAAS works, you actually also know how both ACE inhibitors and ARBs work. ACE inhibitors inhibit the ACE enzyme (of course, it’s in the name). And thus they inhibit the formation of angiotensin II from angiotensin I. Similarly, ARBs—angiotensin receptor blockers—ensure that angiotensin II can’t perform its actions by blocking the receptor to which angiotensin II is supposed to bind.
Both ACE inhibitors and ARBs are found to have similar effects on lowering blood pressure. Cochrane meta-analyses found a decrease in systolic blood pressure by 8 mmHg and diastolic blood pressure by 5 mmHg in the treatment of primary hypertension in both [12, 13]. Half the manufacturer’s maximum recommended daily dose achieved a blood pressure-lowering effect that was 90 % of the maximum dose in the case of ACE inhibitors, and 80 % of the maximum dose in the case of ARBs. As such, increasing the dosage of these drugs usually only lead to very modest further reductions in blood pressure. It then becomes more attractive to combine it with a thiazide-like diuretic. But, something which I would like to highlight right now, if you need multiple drugs to lower your blood pressure sufficiently, I would really urge you to do this under the supervision of a physician.
While both drugs are quite safe and are well-tolerated in general, they, like any drug, can have side effects. This includes first-dose hypotension/dizziness, acute renal impairment, hyperkalemia, coughing, skin rashes, taste disturbance (dysgeusia), hepatotoxicity and angioedema for ACE inhibitors [14]. First-dose hypotension refers to the sudden drop in blood pressure that can occur in the early stages of treatment. Keep this into account with stuff like driving, etc. This effect is exacerbated when you’re dehydrated (for competition or when you’re using a diuretic for whatever reason). Don’t use it at these times. The acute renal impairment occurs in some patients, but usually doesn’t lead to any clinical signs. This is not permanent, once you stop, renal function returns to normal. Again, dehydration is an extra risk factor here. For this purpose, serum creatinine should be measured over time (or other, perhaps more reliable, markers that are used to estimate the glomerular filtration rate [GFR]).
Hyperkalemia (too high potassium in the blood) is quite rare to develop if this is the sole drug you’re using, but the combination with other potassium-sparing drugs, a very high potassium intake from the diet, or existing renal impairment, can increase the risk. As such, in addition to a measurement for renal function, also measure serum electrolytes. Lower the dosage (if possible) if you’re hyperkalemic, or switch to thiazide-like diuretics.
Perhaps the most characteristic side effect of ACE inhibitors is a dry, irritating cough. It occurs roughly in 1 in 10 people [15], and seemingly markedly more in Asians [16]. Occasionally, some people also develop a skin rash from ACE inhibitors [17]. Again, lower the dosage if this occurs and sometimes switching from one ACE inhibitor to another solves this (especially switching away from captopril). And in some very rare cases, cholestasis, cholestatic hepatitis, or hepatocellular injury seem to occur [18]. But there are only some limited case reports about this in the literature.
A final side effect I would like to highlight is angioedema: the accumulation of fluid under the skin or mucous membranes. This might include the face, oral mucosa, tongue, lips, and also the pharynx and larynx [19]. Depending on the location this occurs, it can cause a life-threatening situation by blocking the airways. The incidence of this, however, is pretty low. A meta-analysis found an incidence of 0.3 % vs 0.07 % in the placebo [20]. This side effect doesn’t need to occur in the initial stages of usage, it can sometimes even occur after using it for years. The recommendation is to completely stop using any ACE inhibitor whenever angioedema occurs, and never use it again.
The nice thing about ARBs is that they don’t cause coughing like ACE inhibitors do [21], and they don’t appear to increase the risk of angioedema either [20]. Nevertheless, there’s still the increased risk of hypotension, hyperkalemia and renal dysfunction [21]. Either way, its adherence rate is higher than for any other antihypertensive drug class [22]. Given this and the fact that a recent meta-analysis found no differences between ACE inhibitors and ARBs in terms of blood pressure reduction, fatal events from any cause and cardiovascular causes, fatal and nonfatal myocardial infarctions and strokes [23], ARBs seem the more likely choice of the two, if available.
Commonly prescribed ACE inhibitors are benazepril, captopril, enalapril, fosinopril, lisinopril, perindopril, quinapril, ramipril, and zofenopril. Commonly prescribed ARBs are candesartan, eprosartan, irbesartan, losartan, olmesartan, telmisartan, and valsartan. The Cochrane reviews I mentioned earlier found no ACE inhibitor performing better or worse than any other, and the same goes for the ARBs. There might be some exceptions to this in certain populations (e.g. diabetics), but in general it boils down to whatever you can get your hands on. In my country, the most prescribed ACE inhibitors are captopril, enalapril and lisinopril, and the most prescribed ARBs are valsartan, candesartan and losartan. In general, half the maximum recommended dose of the manufacturer is a good starting dose. Do a blood test before and one month after starting (or after an increase in dose), and if everything comes back normal, every half a year. Include creatinine, eGFR and electrolytes. (There are a lot of guidelines with regard to monitoring, but there isn’t too much consensus about it [24].) Please keep in mind that it takes about 4 to 6 weeks for the full effect of treatment. Ideally you reach a blood pressure below or equal to 130/80 mmHg (but above 120 mmHg).
The thiazide-like diuretics inhibit the action of sodium-chloride symporters in the lumen of the distal tubule of the kidney’s nephrons. Nephrons are the building blocks of the kidneys—the basic functioning unit. Each of them (and your kidneys contain several hundred thousand of them) contributes a tiny bit to the cumulative filtering function of the kidneys. Take a look at the picture below to get an idea of how this looks.
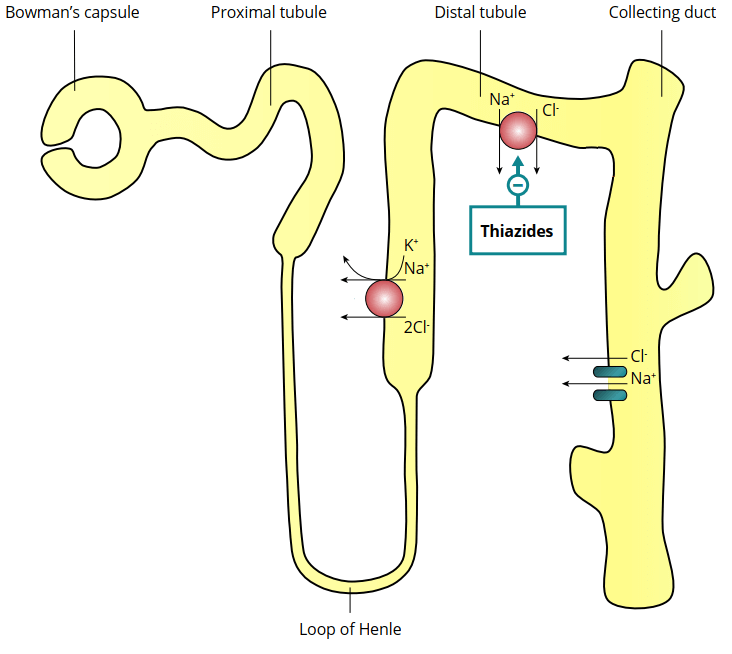
Blood gets squeezed through a cluster of ‘specialized’ capillaries called the glomerulus and the filtrate is then captured in a cup-like sack which surrounds it called Bowman’s capsule. The filter then enters the renal tubule to be processed into urine. Along the way, various substances get reabsorbed from the filtrate into the blood and secreted from the blood into the filtrate.
The symporters upon which thiazide-like diuretics act transport sodium and chloride out of the filtrate. Thus, blocking this symporter means more sodium chloride remains in the filtrate, and therefore more water ends up being excreted. Or in other words, thiazide-like diuretics owe their diuretic (water excreting) action to its natriuretic (sodium excreting) action.
Just as with any diuretic that leads to increased sodium concentrations reaching the distal part of the distal tubule, potassium loss will occur. This is the case because more sodium will get absorbed there and ends up exchanged for potassium (which thus gets secreted into the filtrate). As such, contrary to ARBs and ACE inhibitors, hypokalemia (too low potassium in the blood) can occur. If hypokalemia develops, it’s usually mild but it does highlight the need to test electrolytes. A mild hypokalemia (3.0-3.5 mmol/L) rarely leads to any symptoms. So you won’t notice this. However, if it gets more severe (<2.5-3.0 mmol/L), symptoms such as generalized weakness, fatigue and constipation can develop [25]. Very low potassium levels can also evoke cardiac arrhythmias. In the context of PED-using bodybuilders, several other compounds can contribute to the development of hypokalemia. This includes beta-agonists like clenbuterol, but also high doses of insulin. This can cause a transient shift of potassium from the extracellular compartment to the intracellular compartment. While such a shift only lasts a couple of hours, the effect can be quite drastic in the context of existing hypokalemia. Moreover, caffeine intake can profoundly contribute as well. So also have a look at any pre-workout supplements you’re using, since a 0.26 mmol/L decrease can be observed 4 hours after a 180 mg dose of caffeine, and an even greater increase of 0.44 mmol/L after 360 mg [26]. Either way, if your blood results show mild hypokalemia, you can increase the potassium intake from your diet.
As with any natriuretic, hyponatremia (low sodium levels) can develop. The risk for it is low, but it can be, of course, quite dangerous [27, 28]. Be wary of nausea, headache, muscle cramps, fatigue, gait disturbances, vomiting, feeling confused and difficulty thinking. Drinking a lot of water can also contribute to developing hyponatremia. So don’t purposely drink liters and liters of water. Hypomagnesemia and hypercalcemia can also occur in some cases.
Measure creatinine, eGFR and electrolytes before and a week after starting. If everything comes back normal, do a second measurement within 4 to 8 weeks, after which you can repeat this every 6 to 12 months [24].
Use, preferably, chlorthalidone or indapamide and start with a low dosage such as 12.5 mg chlorthalidone daily or 1.25 mg indapamide daily (although these usually come as 2.5 mg without a break line). After your second blood measurement comes back normal, and blood pressure is not below 130/80 mmHg, you can consider increasing the dose a bit further (this boils down to doubling the dose to 25 mg and 2.5 mg for chlorthalidone and indapamide, respectively). Redo your bloods a week after this dose increase.
Conclusion
That was a long read! But now you know how you measure your blood pressure properly, when you should treat it, and how you should treat it. Treat when it’s above 135/85 mmHg when measured at home, and do this with ARBs, ACE inhibitors or thiazide-like diuretics. In general I would go with ARBs, since these are usually tolerated best. As a final note to this article, I would, again, strongly urge you to get your physician to treat your blood pressure rather than doing this yourself.
Hit up the forums if you’ve got some lingering questions.
References
- Prospective Studies Collaboration. “Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies.” The Lancet 360.9349 (2002): 1903-1913.
- Fonseca-Reyes, Salvador, et al. “Differences and effects of medium and large adult cuffs on blood pressure readings in individuals with muscular arms.” Blood pressure monitoring 14.4 (2009): 166-171.
- Fonseca-Reyes, Salvador, et al. “Effect of standard cuff on blood pressure readings in patients with obese arms. How frequent are arms of a ‘large circumference’?.” Blood pressure monitoring 8.3 (2003): 101-106.
- Unger, Thomas, et al. “2020 International Society of Hypertension global hypertension practice guidelines.” Hypertension 75.6 (2020): 1334-1357.
- Parati, Gianfranco, et al. “European Society of Hypertension practice guidelines for home blood pressure monitoring.” Journal of human hypertension 24.12 (2010): 779-785.
- Brunström, Mattias, and Bo Carlberg. “Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis.” JAMA internal medicine 178.1 (2018): 28-36.
- Ettehad, Dena, et al. “Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis.” The Lancet 387.10022 (2016): 957-967.
- He, Feng J., and Graham A. MacGregor. “Salt, blood pressure and cardiovascular disease.” Current opinion in cardiology 22.4 (2007): 298-305.
- He, Feng J., Jiafu Li, and Graham A. MacGregor. “Effect of longer‐term modest salt reduction on blood pressure.” Cochrane database of systematic reviews 4 (2013).
- Mente, Andrew, et al. “Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies.” The Lancet 388.10043 (2016): 465-475.
- Cushman, William C., et al. “Prevention and Treatment of Hypertension Study (PATHS): effects of an alcohol treatment program on blood pressure.” Archives of internal medicine 158.11 (1998): 1197-1207.
- Heran, Balraj S., et al. “Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension.” Cochrane Database of Systematic Reviews 4 (2008).
- Heran, Balraj S., et al. “Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension.” Cochrane Database of Systematic Reviews 4 (2008).
- Alderman, Christopher P. “Adverse effects of the angiotensin-converting enzyme inhibitors.” Annals of Pharmacotherapy 30.1 (1996): 55-61.
- Bangalore, Sripal, Sunil Kumar, and Franz H. Messerli. “Angiotensin-converting enzyme inhibitor associated cough: deceptive information from the Physicians’ Desk Reference.” The American journal of medicine 123.11 (2010): 1016-1030.
- Tseng, Daniel S., et al. “Angiotensin-converting enzyme-related cough among Chinese-Americans.” The American journal of medicine 123.2 (2010): 183-e11.
- Weber, Michael A. “Safety issues during antihypertensive treatment with angiotensin converting enzyme inhibitors.” The American journal of medicine 84 (1988): 16-23.
- Chitturi, Shivakumar, and Jacob George. “Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs.” Seminars in liver disease. Vol. 22. No. 02. Copyright© 2002 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New York, NY 10001, USA. Tel.:+ 1 (212) 584-4662, 2002.
- Campo, Paloma, et al. “Angioedema induced by angiotensin-converting enzyme inhibitors.” Current opinion in allergy and clinical immunology 13.4 (2013): 337-344.
- Makani, Harikrishna, et al. “Meta-analysis of randomized trials of angioedema as an adverse event of renin–angiotensin system inhibitors.” The American journal of cardiology 110.3 (2012): 383-391.
- Caldeira, Daniel, Cláudio David, and Cristina Sampaio. “Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors.” American Journal of Cardiovascular Drugs 12.4 (2012): 263-277.
- Kronish, Ian M., et al. “Meta-analysis: impact of drug class on adherence to antihypertensives.” Circulation 123.15 (2011): 1611-1621.
- Dimou, Chrisa, et al. “A systematic review and network meta-analysis of the comparative efficacy of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in hypertension.” Journal of human hypertension 33.3 (2019): 188-201.
- McDowell, Sarah E., et al. “A practical guide to monitoring for adverse drug reactions during antihypertensive drug therapy.” Journal of the Royal Society of Medicine 106.3 (2013): 87-119.
- Gennari, F. John. “Hypokalemia.” New England Journal of Medicine 339.7 (1998): 451-458.
- Passmore, A. P., G. B. Kondowe, and G. D. Johnston. “Caffeine and hypokalemia.” Annals of internal medicine 105.3 (1986): 468-468.
- Rodenburg, Eline M., et al. “Thiazide-associated hyponatremia: a population-based study.” American journal of kidney diseases 62.1 (2013): 67-72.
- Spital, Aaron. “Diuretic-induced hyponatremia.” American journal of nephrology 19.4 (1999): 447-452.
Main image by Meine Reise geht hier leider zu Ende. Märchen beginnen mit from Pixabay
About the author
Peter Bond is a scientific author with publications on anabolic steroids, the regulation of an important molecular pathway of muscle growth (mTORC1), and the dietary supplement phosphatidic acid. He is the author of several books in Dutch and English, including Book on Steroids and Bond's Dietary Supplements.

Leave a Reply
You must be logged in to post a comment.