An often requested blood parameter by anabolic steroid users is, not surprisingly, testosterone. However, there appear to be quite some misconceptions about how to interpret these values. People often interpret these values as being very precise. But they aren’t. The results of blood measurements can vary considerably, without the mean steady state values of testosterone over time actually fluctuating. In part this is the result of assay variation: the techniques that are used to measure testosterone can, and often will, yield different values even if you test the same sample twice. They simply aren’t perfect, especially not the commonly used immunoassays—which can also be affected by the presence of other exogenously administered anabolic steroids. In this article I’ll elaborate on how immunoassays work, so that their results can be better appreciated. Moreover, testosterone levels simply fluctuate. I will show in this article how there’s a considerable biological variation that results in differences between two testosterone measurements taken days or several months apart.
Measuring serum testosterone levels: immunoassay
A commonly employed way of measuring testosterone is by means of an immunoassay. This method is cheap and can be automated, making it attractive for commercial labs to provide this option. Usually, this is the way testosterone is measured if you get it tested (unless you explicitly opt for the other option I’ll be covering further down).
An immunoassay can be conducted in various ways, but the key principles are largely the same and the ones measuring steroid hormones are based on competitive binding to antibodies. I’ll be simplifying some things here and there for the sake of brevity: please don’t burn me for it.
So your blood gets drawn, and now you’d want to know how much testosterone is in it. More specifically, you’d like to know the concentration of testosterone in it. Thus, an immunoassay needs to capture the testosterone somehow, while ignoring all the rest that’s in your blood. An immunoassay does this by means of antibodies. Antibodies are molecules that bind very specifically to a certain molecule. Thus capturing the molecule of interest, while ignoring all the rest (there are some caveats to this, though).
So how does this work? Essentially you add the sample to a special piece of plastic that’s covered in antibodies. This plastic surface that’s covered with antibodies is also referred to as the solid phase. And as said, these antibodies that cover the plastic surface are very selective in what they bind and don’t bind. The ideal antibody for an immunoassay binds one thing and one thing only. In the case of a testosterone immunoassay, it binds testosterone and nothing else. So this way it can capture the testosterone from your blood sample, while ignoring all the rest.
But capturing is one thing, so how do you move on to measure it? How do you obtain a concentration value? For this purpose, a signal needs to be generated. A signal that can be measured. So what’s done with these so-called competitive immunoassays is that a known amount of a labeled tracer is added to the sample. And this tracer is the thing that gives off a signal that can be measured. A key property of this labeled tracer is that it binds to the antibodies—the same ones as where the testosterone will bind to—indirectly proportional to the concentration of testosterone in the sample! This is where the competition comes from. On the one hand you got the testosterone from the sample that’s binding to the limited(!) amount of antibodies, and on the other hand you got the labeled tracer that’s doing the same thing. They both want to bind the same antibodies: they’re competing for it. The higher the concentration of testosterone, the more of it will bind to the limited amount of antibodies and thus the less labeled tracer that can be bound to it. And vice versa.
Once you have waited a little bit, for all the binding to have taken place, you ‘wash off’ the solid phase, so you’re left with only the antibodies and whatever is bound to them—testosterone and the labeled tracer.
And with what’s left, you can measure the signal that’s coming off the labeled tracer. The more labeled tracer, the higher the signal, and thus the lower the testosterone concentration must’ve been. After all, there’s a limited amount of antibodies to bind to. So if there’s a lot of testosterone, it will outcompete the labeled tracer to bind to the same antibodies. Illustrated it looks like this:
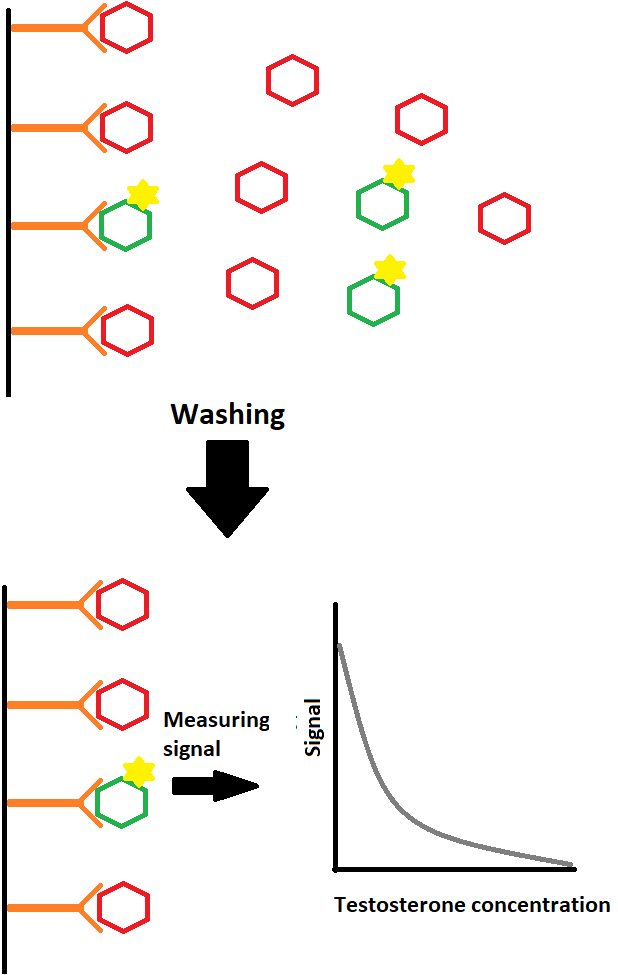
The orange stuff are the antibodies attached to the plastic surface (black vertical line), aka the solid phase. The sample contains testosterone (in red) to which labeled testosterone (in green) of known amount is added. After they have bound to the antibodies you wash off the unbound stuff and measure the remaining signal, i.e. the amount of labeled testosterone bound to the testosterone antibodies. The lower the signal, the higher the testosterone concentration. You can derive the testosterone concentration by looking at a calibration curve of signal strength and testosterone concentration that’s been made with known concentrations of testosterone.
Since there’s a limited amount of antibodies, the testosterone concentration cannot be accurately quantified when someone is injecting large amounts of testosterone. Beyond a certain concentration of testosterone, (almost) all antibodies will be bound by testosterone anyhow. So this is the upper limit of the testosterone concentration the immunoassay can measure (commonly around 60 nmol/L in practice). This can be circumvented, to some degree, by diluting the sample—thus decreasing the concentration, but this needs to be specifically requested.
Another problem with immunoassays is that the antibodies, unfortunately, aren’t absolutely perfect. They commonly also bind to other molecules, which are similar in structure, to some degree or another. Under physiological conditions, these other molecules usually aren’t present in a concentration that’s high enough to significantly impact the test results. However, things are different when you’re injecting a cocktail of various anabolic steroids into your body—steroids with similar chemical structure that will also be present in your blood in high concentrations. These can bind and thereby affect the measurement, this is known as cross-reactivity. For example, methyltestosterone, boldenone, and nandrolone have been found to cross-react in a testosterone immunoassay by Roche [1]. This means that these steroids will, just like testosterone, decrease the signal, and thus lead to—falsely—measured elevated testosterone levels. Of course, per molecule they don’t affect the signal to the same degree as testosterone does. It’s usually only a few percent of testosterone. But a few percent becomes quite considerable if you inject large dosages. Moreover, cross-reactivity is largely unknown for a lot of anabolic steroids for various assays: it might not be too surprising if a certain anabolic steroid demonstrates considerable cross-reactivity that’s tens of percents rather than a few percent.
Immunoassays are often imprecise and inaccurate
In 2007, results were published of the quality-control program by the College of American Pathologists (CAP) [2]. Herein, they had sent blinded samples to over a 1000 labs. (They weren’t actual blood samples, and I suspect that the results would be a bit less precise and accurate if they were.) They sent three different samples to these labs. One sample having the testosterone concentration expected for a normal woman, another for a hypogonadal man and one for a normal man. The 1000+ laboratories ended up with mean values of 33, 97 and 465 ng/dL for each of these samples. So far, so good, that sounds about right. However, there was a marked variability in these measurements and I’ll be focusing on the latter two samples.
The lowest measured value of the hypogonadal sample was 45 ng/dL, whereas the highest was 365 ng/dL. That’s an 8-fold difference! Now, of course, that doesn’t say much. If you get something tested by a billion laboratories you’ll invariably end up with a couple of (extreme) outliers. It’s better to look at the standard deviation, which was 31 ng/dL. In practical terms this roughly means that in about 1 in 4 tests you get a value below 66 ng/dL or above 128 ng/dL. This also doesn’t sound much, but percentually this is simply a big difference. Luckily this doesn’t matter much in clinical practice, as in both cases you’re clearly hypogonadal.
But what about the other sample that should be representative of a normal man? The one with a mean measured value of 465 ng/dL. Here things get a little more tricky. The lowest measured value was 276 ng/dL, which, depending on symptoms, can be considered to be in the hypogonadal range. The highest measured value was 744 ng/dL. The standard deviation was 81 ng/dL. Implying that about 1 in 4 tests would have this man below 384 ng/dL or above 546 ng/dL.
These differences are the result of a variety of reasons. One being that immunoassays simply aren’t perfect. You can test the exact same sample on the exact same device and get different results. Another reason is that one lab might have calibrated its device differently than the other, thus leading to different results with the same sample, the same device, but between different labs. Yet another reason is that the devices used to measure testosterone vary from one lab to another. One uses the Bayer X, another uses the Roche Y, yet another uses the DPC Z, etc. This should always be kept in mind when interpreting testosterone values from immunoassays. If you measure 400 ng/dL (13.9 nmol/L) today, and 500 ng/dL (17.4 nmol/L) a month later, this certainly doesn’t mean that your testosterone has increased. It could be! But it simply also could be the result of assay variability: measurement imprecision or error. (Or, of course, a bit of both.)
For those interested, here are the results for the two samples from the paper:

Testosterone measurements also vary because of biological variation
Your testosterone levels aren’t static. They vary over time. In a sense, they oscillate around a certain value. One study investigated the biological and assay variation of various hormones, including testosterone, and found the biological variation to be larger than the assay variation [3]. To be clear: they used an immunoassay to determine testosterone levels in this study. Subjects were excluded in this study if they used any medications that alter hormone levels. Additionally, they ensured sampling was being done within 4 hours of a subject’s awakening, and sampling was postponed to another day if the time of awakening was substantially different from a subject’s normal pattern. Since testosterone levels follow a diurnal pattern, i.e. highest levels around awakening with a through nearing the end of the day, biological variation will be an even bigger factor if this isn’t accounted for.
Together, these variations can lead to vastly different results between measurements. The percentage difference that would be exceeded half the time(!) between two measurements of testosterone was roughly 25 %. So if you measure 575 ng/dL (20 nmol/L) today, there’s a 50 % chance that the next measurement will be less than 460 ng/dL (16 nmol/L) or more than 720 ng/dL (25 nmol/L) as a result of assay and biological variation. Considerably bigger differences will occur too, but less frequently.
These variations in testosterone will be smaller, but still significantly present, with more precise measurements such as those performed with liquid chromatography tandem mass spectrometry (LC-MS/MS). After all, over half the variation appears to be the result of biological variation. So always keep this in mind when interpreting blood values.
Conclusion
It’s hard to say something about small-to-moderate variations between testosterone measurements of an individual. Assay and biological variation simply lead to varying results between measurements. A drop between measurements doesn’t necessarily mean your testosterone is deteriorating and neither does an increase necessarily mean that it’s improving. The amplitude of the change and the mean value over several tests over time should be taken into account, as well as clinical signs of hypogonadism when testosterone deficiency is suspected.
References
- Krasowski, Matthew D., et al. “Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction.” BMC clinical pathology 14.1 (2014): 1-13.
- Cao, Zhimin Tim, et al. “Accuracy-based proficiency testing for testosterone measurements with immunoassays and liquid chromatography-mass spectrometry.” Clinica Chimica Acta 469 (2017): 31-36.
- Brambilla, Donald J., et al. “Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men.” Clinical endocrinology 67.6 (2007): 853-862.
About the author
Peter Bond is a scientific author with publications on anabolic steroids, the regulation of an important molecular pathway of muscle growth (mTORC1), and the dietary supplement phosphatidic acid. He is the author of several books in Dutch and English, including Book on Steroids and Bond's Dietary Supplements.

Leave a Reply
You must be logged in to post a comment.