
Estimated reading time: 1 minute
Table of contents
Introduction
MENT (trestolone; methylnortestosterone; 7α-methyl-19-nortestosterone; 7α-CH₃-NorT) is a non-5α-reducible androst-4-ene-3-one, potently progestagenic and estrogenic anabolic-androgenic steroid (AAS). MENT is non-17α-methylated mibolerone (Cheque drops™; 7α,17α-CH₃-NorT). MENT’s unique features do not imbue it with any special bodybuilding use case, unlike the prior AAS that this author has reviewed (e.g., trenbolone for recomp, stanozolol for drying-out, fluoxymesterone for peaking). Rather, its features combine unfavorably for any bodybuilding practical use, across the dimensions of gynecomastia (causing male breast tissue growth), fluid balance (causing “bloat”), and blood pressure (causing systolic hypertension or increased pulse pressure).
This is part four of an 11-part series on the unique characteristics of various AAS.
Combinations – Types of Effects:
Additive: Effects that typically act via the same receptor(s) and/or mechanism(s) on the same end-point (e.g., increased muscle protein synthesis via the AR). To apply a heuristic, these are effects for which 1 + 1 = 2.
Synergistic (Greater than Additive): Effects that typically act via different receptor(s) and/or mechanism(s) on the same end-point (e.g., decreased muscle protein breakdown via decreased GR number and antagonism of GR). To apply a heuristic, these are 1 + 1 > 2
MENT’s Unique Features
- Estrogenic Effects by aromatization to the most potent known aromatic product of any known AAS – 7α-methylestradiol (7α-ME)
- Gestagenic Effects by strong progesterone receptor (PR) activation:
- That combine synergistically to yield:
- Gynecomastic Effects (growth of breast tissue in men)
- Edematous Effects (fluid retention; “bloat”), and
- Hypertensive Effects (elevated blood pressure; particularly systolic; i.e., pulse pressure)
- That combine synergistically to yield:
Before we can discuss MENT’s uniquely Edematous Effects (fluid retentive; “bloating”) we must first iterate through the factors that underpin fluid retention (Estrogenic Effects and Gestagenic Effects) first before next discussing how those same factors underpin its particular Hypertensive Effects (elevated blood pressure, particularly systolic; i.e., increased pulse pressure).
Estrogenic Effects
MENT uniquely aromatizes to the most potent known aromatic product of any AAS – 7α-methylestradiol (7α-ME). [1].
From the The Theory of Compound-Dependent (Per-AAS) and Individualized (Per-User) Estrogenic Potencies (Author’s Model), estrogenicity refers to the effects associated with ER- α and β activation (the latter generally opposing the former), and depends on Compound-Dependent (Per-AAS) and Individualized (Per-User) Factors that determine both (A) actual blood levels of, and (B) tissue-level effects of, an AAS’s aromatic products.
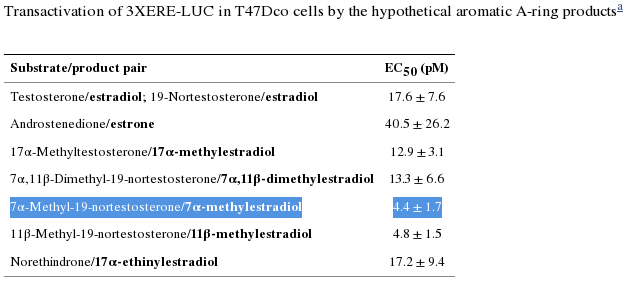
Gynecomastic Effects Arising Out of Estrogenicity
MENT’s aromatic product, 7α-ME, is more than fourfold the potency (“efficacious,” a bad thing here) as estradiol (E2) in ER-containing cells. [1]. Efficacy is determined by measuring the effect, e.g., growth (here, in breast cancer cells). The EC₅₀ (EC50) is determined by the concentrations at which the ligand triggers growth and may be confirmed by measurements of cell cycle progression (i.e., the S-phase entry during the cell cycle).
The binding affinity (IC₅₀) of MENT’s aromatic product, 7α-ME, is 102% that of E2, which is typically used as the reference compound for ER binding given its noteworthy efficacy, potency, and affinity for the ER-α receptor, in the literature. [1].
Comparing the rate of aromatization between MENT and nandrolone, Attardi and colleagues found that, “[a]t 180 min, about 23% of MENT was converted to 7α-ME and about 13% of [nandrolone] to E2.” Since nandrolone aromatizes at 20% the rate of testosterone (T), we can deduce that MENT aromatizes at roughly 35% the rate of T to 7α-ME, with its four-fold potency versus E2, i.e., for causing growth in breast cancer cells. Simple multiplication of the rate of aromatization (35%) × EC50(7α-methylestradiol) × RBA(7α-methylestradiol) ≈ MENT’s 40% greater growth potential in ER-containing cells than T. [2]. †.
Deduction, then, supports reports by users that MENT is potently estrogenic. Mathematically, we are able to say that 50 mg daily of MENT E ≈ as estrogenic as 500 mg of Test E weekly.
Further, as described in the following section – MENT’s Gestagenic Effects dramatically potentiates its hypertensive and edematous (proclivity to retain fluid) effects.
Gestagenic Effects
Gynecomastic Effects Arising Out of Antiandrogenicity
MENT is a potently progestagenic (“gestagenic”) androgen that possesses 27.5% the potency of Androcur™ – an anti-androgen and progestin medication used to treat androgen-dependent maladies including acne, hirsutism, and prostate cancer – to activate the progesterone receptor (PR). [11].
Progestins contribute to hypothalamo-pituitary gonadal (HPG) axis suppression by dysregulating hypothalamic gonadotropins via KNDy dendron signalling, disrupting GnRH pulsatility, and inhibiting pituitary LH secretion, thereby inhibiting (endogenous) T synthesis and secretion. [12]. [13]. Synthetic progestins used in contraception derive efficacy from this feature. Bebb and colleagues randomized healthy men to receive either testosterone enanthate (100 mg weekly), or the same dosage of testosterone in combination with the progestin levonorgestrel, the addition of which virtually abolished LH and FSH secretion. [14]. Decreased LH and FSH can cause secondary hypogonadism, thereby decreasing the androgen/estrogen ratio (A:E), causing gynecomastia. [15].
The effects of progestins relate to their interactions with receptors: androgen receptors (AR) (e.g., acnea, lipid effects); glucocorticoid receptors (GR) (eg., salt and water retention, bloating); or mineralocorticoid receptors (MR) (e.g., decreased water retention and weight). Anti-androgenic progestins may act in several ways. They can exert competitive inhibition of the AR, or bind to the enzyme 5-alpha reductase and hence interact with the conversion of testosterone into dihydrotestosterone (its active metabolite).[16].
Progesterone and its derivatives, and progesterone mimics (e.g., MENT) moderately bind to the AR in a competitive (i.e., antagonist) mode. [17]. Progesterone derivatives alter both AR- and PR-, but not ER-, mediated tissue responses. [17].
Estrogens up-regulate PR synthesis. [18]. Further, activation of PR has been linked to reduced expression of AR, thereby hampering the androgen-mediated inhibition on breast tissue growth observed in condition of normal hormonal homeostasis. [19].
Progesterone and its derivatives may further cause gynecomastia by enhancing the effect of E2 on breast tissues. [20].
In summary, the features that have been discusses – MENT’s Estrogenic Effects and Gestagenic Effects – underpin its potent Edematous Effects and its Hypertensive Effects.
Edematous Effects
Edema, or fluid retention, is the touchstone of excessive estrogenicity in AAS-using bodybuilders.
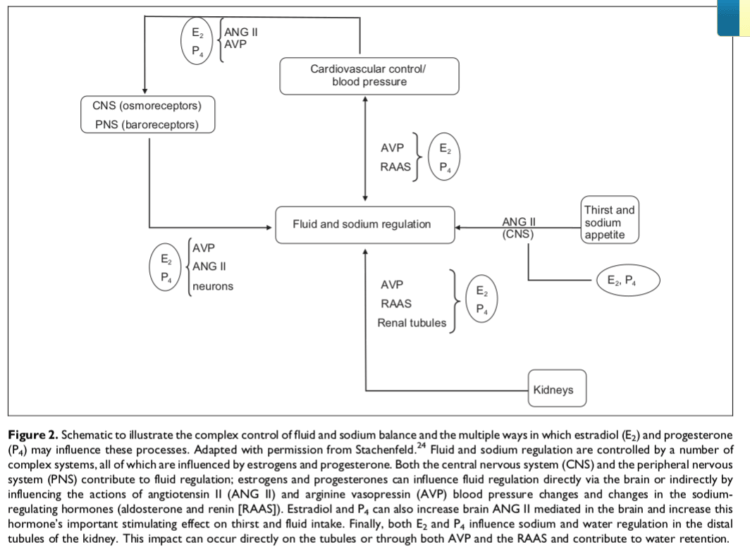
This diagram illustrates how MENT manifoldly acts to promote fluid retention (edema) and hypertension (increased systolic blood pressure; Increase Pulse Pressure). MENT is analogous to E₂ (estradiol; E2) and P₄ (progesterone) vis-à-vis 7α-ME and its 29.2% potency to activate the PR a la P₄. [11].
MENT, then, promotes fluid retention by acting on:
- The brain and central nervous system (CNS) to increase thirst and sodium appetite via angiotensin II signalling (ANG II), and
- The kidneys by acting on:
- antidiuretic hormones, e.g., arginine vasopressin (AVP), reducing urination
- the renin-angiotensin-aldosterone system (RAAS), increasing sodium balance, and/or
- the renal tubuli, promoting sodium and water retention. [10].
Hypertensive Effects
Figure 1 also illustrates, partly, how MENT, standing in the place of E₂ (estradiol; E2) and P₄ (progesterone) promotes Increased Pulse Pressure. It does so by increasing fluid balance (i.e., “bloat”) a la its Edematous Effects and by acting on the osmoreceptors of the central nervous system (CNS) and on the baroreceptors of the peripheral nervous system (PNS), as well as by acting on angiotensin II (ANG II), AVP, and neurons throughout various systems. [10].
Increased Pulse Pressure
MENT significantly increases systolic blood pressure (i.e., pulse pressure) at a weekly dose of less than 2 mg. [21].
Pulse pressure (Pp) is the difference (mmHg) between systolic & diastolic pressures. A normal pulse pressure is, then, 40 mmHg (120 mmHg – 80 mmHg = 40 mmHg).
Pulse Pressure
Pulse pressure (Pp) represents the force that the heart generates each time it contracts, or arterial compliance (C). Where pulse pressure is normal at 40 mmHg, a Pp < 25% of the systolic pressure is low or narrowed, and a Pp > 100 mmHg is high or widened.
A change in pulse pressure (ΔPp) is proportional to volume (V) change (ΔV) but inversely proportional to arterial compliance (C):
ΔPp = Δ V/C
Because the change in volume is due to the stroke volume (SV) of blood ejected from the left ventricle, we can approximate pulse pressure as:
Pp = SV/C
A normal young adult at rest has a stroke volume (SV) of approximately 80 mL. Arterial compliance (C) is approximately 2 mL/mmHg, which confirms that normal pulse pressure is approximately 40 mmHg.
MENT, therefore induces a widened pulse pressure, increasing stroke volume.
Haematologic Effects
Blood Thickening
MENT, by increasing sodium and fluid retention, increases plasma volume. It also increases hemoglobin rapidly.
Hemoglobin and Hematocrit
Hemoglobin (Hb) is a binding protein in erythrocytes (RBCs) for O₂ (Hb 13.5 – 17 g/dL [men], 12 – 15.5 g/dL [women])
Hematocrit (HCT) represents the % of blood volume occupied by erythrocytes (RBCs) [men 41-51%, women 36-47%].
Hematocrit (HCT) is related to hemoglobin (Hb) by the basic formula:
Hb (g/dL) × 3 ≈ HCT (%)
Example: Hb of 15 g/dL ≈ HCT of 45%.
Hemoglobin levels were significantly increased (149 ± 2.9 g/L → 154 ± 3.3; effect size: 1.724; %Δ: +3.35%; 95% confidence interval) by MENT (~ 2 mg q.w.) at 12 weeks, and a similar pattern (0.44 to 0.46; effect size: 2; %Δ: +4.5%; not significant) was seen in hematocrit, but this did not reach statistical significance. [21]. In the testosterone group (~ 120 mg q.w.), by contrast, a slower progressive rise in hemoglobin concentration was observed that only became significant at 48 weeks. There was also a significant overall rise in hematocrit in the testosterone group, although none of the individual treatment time points were significantly different from pre-treatment (0.45 ± 0.01). [21].
Conclusion
MENT’s basic features – its potent estro- and gesta- genicity – lay the basic foundation for its sharp Edematous Effects and Hypertensive Effects in a way that its gynecomastic effects, those Gynecomastic Effects Arising Out of Estrogenicity and Gynecomastic Effects Arising Out of Antiandrogenicity, cannot be ignored. In the mode of 7α-ME, it potently suppresses gonadotropins (LH, FSH), decreasing the A:E, synergizing with gestagenic features with potency on par with pharmaceutical antiandrogens (e.g., Androcur™), stimulating growth directly in breast tissue via ER, and indirectly via gestagenic and antiandrogenic action in the mode of progesterone (with nearly ⅓ potency per-mg). MENT particularly promotes “bloat” via its action on the kidneys (regulating fluid and sodium retention) and brain (increasing thirst and sodium ingestion) and particularly promotes hypertension, especially increased pulse pressure, by increasing stroke volume via augmented plasma volume and hematocrit, i.e., blood viscosity, or thickness.
This article might foreseeable serve as a deterrent to bodybuilders’ use of this practically useless agent.
Still, it does have some unique(ly terrible) aspects.
Endnotes
† We must also consider the rate of breakdown of the produced 7a-methylestradiol, as well as the estrogenic potency of the resulting metabolites (Peter Bond, Oct 20 2021).
References
[1] Attardi BJ, Pham TC, Radler LC, Burgenson J, Hild SA, Reel JR. Dimethandrolone (7alpha,11beta-dimethyl-19-nortestosterone) and 11beta-methyl-19-nortestosterone are not converted to aromatic A-ring products in the presence of recombinant human aromatase. J Steroid Biochem Mol Biol. 2008;110(3-5):214-222. doi:10.1016/j.jsbmb.2007.11.009
[2] Ryan, Kenneth J. “Biological aromatization of steroids.” Journal of Biological Chemistry 234.2. (1959): 268-272.
[3] Navarro, V. M., Gottsch, M. L., Chavkin, C., Okamura, H., Clifton, D. K., and Steiner, R. A. (2009). Regulation of Gonadotropin-Releasing Hormone Secretion by Kisspeptin/Dynorphin/Neurokinin B Neurons in the Arcuate Nucleus of the Mouse. Journal of Neuroscience, 29(38), 11859–11866. doi:10.1523/jneurosci.1569-09.2009
[4] Girmus, R. L., and Wise, M. E. (1992). Progesterone Directly Inhibits Pituitary Luteinizing Hormone Secretion in an Estradiol-dependent Manner1. Biology of Reproduction, 46(4), 710–714. doi:10.1095/biolreprod46.4.710
[5] Bebb, R. A., Anawalt, B. D., Christensen, R. B., Paulsen, C. A., Bremner, W. J., and Matsumoto, A. M. (1996). Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. The Journal of Clinical Endocrinology and Metabolism, 81(2), 757–762. doi:10.1210/jcem.81.2.8636300
[6] Sitruk-Ware, R. (2004). Pharmacological profile of progestins. Maturitas, 47(4), 277–283. doi:10.1016/j.maturitas.2004.01.001
[7] Fang, H., Tong, W., Branham, W. S., Moland, C. L., Dial, S. L., Hong, H., … Sheehan, D. M. (2003). Study of 202 Natural, Synthetic, and Environmental Chemicals for Binding to the Androgen Receptor. Chemical Research in Toxicology, 16(10), 1338–1358. doi:10.1021/tx030011g
[8] Eyster, K. M. (Ed.). (2016). Estrogen Receptors. Methods in Molecular Biology. doi:10.1007/978-1-4939-3127-9
[9] Sansone, A., Romanelli, F., Sansone, M., Lenzi, A., and Di Luigi, L. (2016). Gynecomastia and hormones. Endocrine, 55(1), 37–44. doi:10.1007/s12020-016-0975-9
[10] Zhou, J., Ng, S., Adesanya-Famuiya, O., Anderson, K., and Bondy, C. A. (2000). Testosterone inhibits estrogen-induced mammary epithelial proliferation and suppresses estrogen receptor expression. The FASEB Journal, 14(12), 1725–1730. doi:10.1096/fj.99-0863com
[11] Houtman, C. J., Sterk, S. S., van de Heijning, M. P. M., Brouwer, A., Stephany, R. W., van der Burg, B., and Sonneveld, E. (2009). Detection of anabolic androgenic steroid abuse in doping control using mammalian reporter gene bioassays. Analytica Chimica Acta, 637(1-2), 247–258. doi:10.1016/j.aca.2008.09.037
[12] Navarro, V. M., Gottsch, M. L., Chavkin, C., Okamura, H., Clifton, D. K., and Steiner, R. A. (2009). Regulation of Gonadotropin-Releasing Hormone Secretion by Kisspeptin/Dynorphin/Neurokinin B Neurons in the Arcuate Nucleus of the Mouse. Journal of Neuroscience, 29(38), 11859–11866. doi:10.1523/jneurosci.1569-09.2009
[13] Girmus, R. L., and Wise, M. E. (1992). Progesterone Directly Inhibits Pituitary Luteinizing Hormone Secretion in an Estradiol-dependent Manner1. Biology of Reproduction, 46(4), 710–714. doi:10.1095/biolreprod46.4.710
[14] Bebb, R. A., Anawalt, B. D., Christensen, R. B., Paulsen, C. A., Bremner, W. J., and Matsumoto, A. M. (1996). Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. The Journal of Clinical Endocrinology and Metabolism, 81(2), 757–762. doi:10.1210/jcem.81.2.8636300
[15] Narula HS, Carlson HE. Gynaecomastia–pathophysiology, diagnosis and treatment. Nat Rev Endocrinol. 2014;10(11):684-698. doi:10.1038/nrendo.2014.139
[16] Sitruk-Ware, R. (2004). Pharmacological profile of progestins. Maturitas, 47(4), 277–283. doi:10.1016/j.maturitas.2004.01.001
[17] Fang, H., Tong, W., Branham, W. S., Moland, C. L., Dial, S. L., Hong, H., … Sheehan, D. M. (2003). Study of 202 Natural, Synthetic, and Environmental Chemicals for Binding to the Androgen Receptor. Chemical Research in Toxicology, 16(10), 1338–1358. doi:10.1021/tx030011g
[18] Eyster, K. M. (Ed.). (2016). Estrogen Receptors. Methods in Molecular Biology. Doi:10.1007/978-1-4939-3127-9
[19] Sansone, A., Romanelli, F., Sansone, M., Lenzi, A., and Di Luigi, L. (2016). Gynecomastia and hormones. Endocrine, 55(1), 37–44. doi:10.1007/s12020-016-0975-9
[20] Zhou, J., Ng, S., Adesanya-Famuiya, O., Anderson, K., and Bondy, C. A. (2000). Testosterone inhibits estrogen-induced mammary epithelial proliferation and suppresses estrogen receptor expression. The FASEB Journal, 14(12), 1725–1730. doi:10.1096/fj.99-0863com
[21] Walton, M. J., Kumar, N., Baird, D. T., Ludlow, H., & Anderson, R. A. (2007). 7 -Methyl-19-Nortestosterone (MENT) vs Testosterone in Combination With Etonogestrel Implants for Spermatogenic Suppression in Healthy Men. Journal of Andrology, 28(5), 679–688. doi:10.2164/jandrol.107.002683
About the author
Type-IIx is a physique coach, author, and researcher. Bolus: A Practical Guide and Reference for recombinant Human Growth Hormone Use will be his first published textbook, anticipated for release in early 2023. Ampouletude.com will be Type-IIx's base of operations for coaching services and publications. Type-IIx is proud to be a contributing writer to MesoRx, his home forum, where he is a regular poster.
Leave a Reply
You must be logged in to post a comment.