
Estimated reading time: 8 minutes
Table of contents
Introduction
Oxandrolone (17β-hydroxy-17α-methyl-2-oxa-5α-androstan-3-one; Anavar; Oxabone®; Oxandrine®; “Var”), a member of the 5α-androstan-3-one class of anabolic-androgenic steroids (AAS), a 2-oxasteroid, is an attenuated androgen, or an AAS whose anabolic features predominate over its androgenic (masculinizing, suppressive, male-pattern balding, etc.) features. Oxandrolone is a nonaromatizable and non-5α-reducible (since it’s already 5α-reduced) AAS.
Oxandrolone is an AAS that confers particular benefits for reducing abdominal fat, anticatabolism (i.e., glucocorticoid modulation by cross-talk regulation between AR and GR), and strength. However, oxandrolone also affects lipids unfavorably. This fact connotes that rational oxandrolone use requires a balancing of considerations (trading-off) of the benefit for reducing abdominal fat weighed against this dyslipidemic risk.
Oxandrolone, while regarded as “weak,” and a “women’s drug” by some detractors, holds prominence among the 17α-alkylated AAS (17AAs) as an effective and well-tolerated drug that sheds ab fat, adds lean tissue during cutting, and boosts strength potently. While true that it is widely used by women for its remarkable tolerability (i.e., attenuated androgenicity) and outstanding potency (e.g., greater than Primo), it is an effective recomp drug with some edge case uses for peaking or hardening in men also.
Abdominal Fat Mass Reduction
Oxandrolone possesses particular lipolytic effects on subcutaneous abdominal fat and a tendency to reduce visceral fat mass. [1]. Indeed, oxandrolone decreases subcutaneous abdominal fat better than testosterone enanthate, and has a tendency to reduce visceral fat not merely more than testosterone enanthate but, also, rather than increasing it unlike nandrolone decanoate (from three- to nine- months). [1]. It follows that for this to be true, oxandrolone must possesses some unique mechanism among the constellation of AAS that imbues it with this body recomposition benefit.
Oxandrolone’s unique advantage for abdominal fat mass reduction is related to its stimulating Hepatic Ketogenesis. [2]. Before we count our chickens (i.e., enhanced hepatic ketogenesis, therefore, enhanced fat mass reduction), though, we must take stock of the reality that this feature is another double-edged sword, analogous to nandrolone’s bimodal effects on connective tissues at the expense of left-ventricular hypertrophic and diastolic function a la its stimulating ACE activity. [3]. The tradeoffs (i.e., dyslipidemia) of this enhanced hepatic ketogenic mechanism must be understood.
Dyslipidemia
Oxandrolone increases hepatic lipase activity unfavorably by reducing larger lipoproteins to smaller ones (causing a shift from HDL-C to VLDL), posing an elevated dyslipidemic risk. [4]. [2]. Very low-density lipoproteins (VLDL), the most atherosclerotic fractions, coagulate in the heart’s “nooks and crannies,” increasing risk of myocardial infarction or heart attack.
Oxandrolone’s lowering of HDL-C is caused by an increased hepatic triglyceride lipase activity (HTGLA). Hepatic lipase (HL) is a liver-secreted enzyme that liberates fatty acids from triacylglycerol and phospholipids that are part of lipoproteins, including HDL, thereby shifting from larger HDL2 to smaller HDL3 particles, susceptible to further breakdown, thereby reducing HDL. [5]. Since oxandrolone is nonaromatizable, it is devoid of any estrogen benefits to lipids (estrogens ↓HTGLA), and since it is metabolized extensively in the liver it exerts profound effects on hepatic proteins (e.g., HL). [6].
Hepatic Ketogenesis
That same increase to HTGLA that diminishes lipid particle size accompanies enhanced hepatic ketogenesis. [2]. Dietary fats are non-water soluble (hydrophobic, lipophilic) and must be digested in the stomach with the aid of bile acids released by the gallbladder, and during fat transport are packaged as chylomicrons via the lymphatic system. [7]. In muscle or fat tissue, they are acted upon by lipoprotein lipase (LPL), which liberates the FAs from the chylomicron: those FAs can either be stored in the adipocyte (fat cell) or taken up into the bloodstream for use by other tissues such as muscle or liver. [7]. Increased lipolysis of triglyceride-rich lipoproteins (e.g., chylomicrons, VLDL) by HL takes FAs to be transported to the liver and enhanced fatty acid oxidation (ketogenesis; marked by increased 3-hydroxybutyrate) depletes fat substrate from the pool that would otherwise be destined for storage in fat cells. [2]. Enhanced ketogenesis, characterized by increased fat substrate utilization, has a knock-on effect – to spare protein (anticatabolic). Whether the FAs are stored or released depends on the energy state of the cell, i.e., deficit vs. surplus.
Glucocorticoid Modulation: Anticatabolism
Oxandrolone Regulates Cross-Talk Between Androgen Receptor (AR) and Glucocorticoid Receptor (GR)
Oxandrolone decreases glucocorticoid receptor (GR) transactivation by increasing androgen receptor (AR) and GR heterodimer (AR-GR) formation, regulating cross-talk between AR and GR, in turn decreasing glucocorticoid activation of GR, exerting anticatabolic effects. [8].
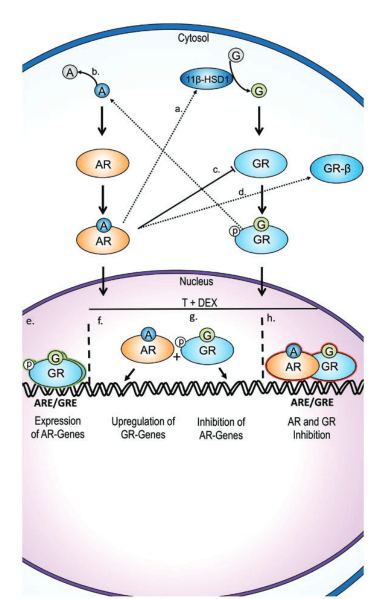
Readers are likely mostly familiar with the classical (canonical) binding of AAS/oxandrolone to AR. The primary mechanism of AR binding is direct (nuclear) gene transcription. The binding of a ligand results in specific conformational changes in the AR’s ligand-binding domain which in turn causes dissociation of heat shock proteins, dimerization and transport from the cytosol to the cell nucleus where the AAS-AR dimer complex binds to AREs (61). [9]. The ligand (e.g., oxandrolone) stimulation of AR transcriptional activity requires AR interaction with a variety of cellular proteins, coregulators, that facilitate AR conformation, nuclear localization, DNA binding, and interaction with the basal transcriptional machinery. [9].
Oxandrolone negatively regulates glucocorticoid (e.g., cortisol) activation of GR at the ligand and receptor level, as well as at the gene expression (i.e., translation, protein synthesis) level, by regulating GR/AR crosstalk. [8]. [10]. This means that oxandrolone, not by competitively inhibiting glucocorticoid binding (GR antagonism) [so unlike fluoxymesterone (Halo)], nor strictly by ARE/GRE chaperones/coregulators or chromatin modification, and certainly not by decreasing GR number [unlike trenbolone], but rather by a mechanism of crosstalk between the AR and GR. Figure 1. [10].
Practically, this crosstalk regulation of catabolic effects by oxandrolone offers yet another AAS feature that offers potential synergy in a pairwise fashion with that subset of AAS whose anticatabolic, glucocorticoid-modulatory effects arise out of distinct non-crosstalk mechanisms (e.g., fluoxymesterone, trenbolone).
Strength
Creatine Synthesis
Oxandrolone particularly stimulates strength, ostensibly due to particularly stimulating creatine synthesis and phosphocreatine (PCr) stores, that replenish ATP for muscle contraction. [11]. [12]. Whereas 17AAs generally stimulate hypercreatinuria (excess creatine in urine), parenteral (i.m.) non-C-17-alkylated AAS do not. [12]. This is hypothesized to occur due to, (a) 17AA-induced creatine synthesis and therefore PCr stores, repleting the body’s energy source for anaerobic/glycolytic efforts like heavy lifting, and/or (b) non-C-17-alkylated (i.e., intramuscularly injected; i.m.) AAS use the pool of amino acids (e.g., glycine, arginine, methionine) that would otherwise be directed to creatine synthesis instead to cell proteins [meaning that injectable non-C-17-alkylated AAS lay down more actual skeletal muscle tissue].
Empirically, oxandrolone’s rapid effects on strength are noteworthy and must occur by a mechanism besides ligand-induced transcription (i.e., canonical AR protein synthesis) because of the short time-course. This effect often surprises men at first impression given oxandrolone’s notoriety as a “women’s drug.” Oxandrolone’s remarkably strength-promoting belies the mythology that androgenic potency is directly related to strength, since it is an attenuated androgen.
Conclusion
Var sheds ab fat for a reason, and is a surprisingly strength-promoting steroid, despite its reduced androgenic potency and commonplace use among women, since it seemingly particularly stimulates creatine phosphate stores. Var’s anticatabolic potency derives from its crosstalk regulation of cortisol; and its melting ab fat is thanks to enhanced hepatic ketogenesis. The biggest downside is it’s harsh on lipids.
References
[1] Lovejoy JC, Bray GA, Greeson CS, Klemperer M, Morris J, Partington C, Tulley R. Oral anabolic steroid treatment, but not parenteral androgen treatment, decreases abdominal fat in obese, older men. Int J Obes Relat Metab Disord. 1995 Sep;19(9):614-24.
[2] Vega, G. L., Clarenbach, J. J., Dunn, F., & Grundy, S. M. (2008). Oxandrolone Enhances Hepatic Ketogenesis in Adult Men. Journal of Investigative Medicine, 56(7), 920–924. doi:10.2310/jim.0b013e318189153d
[3] Mannion, Cormac [Type-IIx]. “Nandrolone Insights: Unique Characteristics of Anabolic-Androgenic Steroids – Part 6.” Meso-Rx, thinksteroids.com. 19 Sep. 2024, https://thinksteroids.com/articles/nandrolone-benefits-side-effects-joint-support-muscle-growth/
[4] Ehnholm C, Huttunen JK, Kinnunen PJ, Miettinen TA, Nikkilä EA. Effect of oxandrolone treatment on the activity of lipoprotein lipase, hepatic lipase and phospholipase A1 of human postheparin plasma. N Engl J Med. 1975;292(25):1314-1317. doi:10.1056/NEJM197506192922503
[5] Bond, Peter. Book on Steroids: A Complete Evidence-Based Reference. PeterBond.org, 2020.
[6] Friedl, K. E., Hannan, C. J., Jones, R. E., & Plymate, S. R. (1990). High-density lipoprotein cholesterol is not decreased if an aromatizable androgen is administered. Metabolism, 39(1), 69–74. doi:10.1016/0026-0495(90)90150-b
[7] McDonald, Lyle. The Ultimate Diet 2.0: Advanced Cyclical Dieting for Achieving Super Leanness. Lyle McDonald Publishing, 2003.
[8] Zhao, J., Bauman, W. A., Huang, R., Caplan, A. J., & Cardozo, C. (2004). Oxandrolone blocks glucocorticoid signaling in an androgen receptor-dependent manner. Steroids, 69(5), 357–366. doi:10.1016/j.steroids.2004.01.006
[9] Fragkaki, A. G., Angelis, Y. S., Koupparis, M., Tsantili-Kakoulidou, A., Kokotos, G., & Georgakopoulos, C. (2009). Structural characteristics of anabolic androgenic steroids contributing to binding to the androgen receptor and to their anabolic and androgenic activities. Steroids, 74(2), 172–197. doi:10.1016/j.steroids.2008.10.016
[10] Ruiz, D., Padmanabhan, V., & Sargis, P.M. (2020). Stress, sex, and sugar: Glucocorticoids and sex-steroid crosstalk to sex-specific misprogramming of metabolism. Endocrine Society. Aug 2020. 4(8). pp. 1 – 28. doi:10.1210/jendo/bvaa087
[11] Duchaine, Daniel. Underground Steroid Handbook II: Update: 1992. Power Distributors ; Dist. by Crain’s Muscle World, 1992.
[12] Krüskemper, Hans Ludwig. Anabolic Steroids. 1968.
About the author
Type-IIx is a physique coach, author, and researcher. Bolus: A Practical Guide and Reference for recombinant Human Growth Hormone Use will be his first published textbook, anticipated for release in early 2023. Ampouletude.com will be Type-IIx's base of operations for coaching services and publications. Type-IIx is proud to be a contributing writer to MesoRx, his home forum, where he is a regular poster.
Leave a Reply
You must be logged in to post a comment.