
The previous three parts of this article series have discussed a variety of topics related to protein and amino acids (AAs): digestion, protein quality, requirements and many others. To help readers better understand some of the mechanisms that regulate protein and AA metabolism in the body, it is necessary to develop a model of AA metabolism.
While all the details of the model have not yet been elucidated, the concepts presented should give a general overview of the possible routes that proteins may take once ingested. Additionally, the parts of the model which are affected by high- and low-caloric intake, as well as high- and low-protein intake (e.g. protein cycling) are discussed.
Section 7: A model of amino acid kinetics
Homeostasis
Before building a model of protein kinetics, readers need to understand the concept of homeostasis. With few exceptions, the body tries it’s best to maintain body protein stores at constant levels (1). In addition, the body attempts to maintain a pattern of constancy in the free AA pool and in the rate of protein turnover (1). The regulatory mechanisms responsible are discussed shortly.
Arguably the primary exception to homeostasis is the performance of resistance training which stimulates the body to increase protein stores above habitual levels. In contrast, excessive aerobic exercise tends to decrease and maintain protein stores below normal levels.
To maintain homeostasis, the body has four major systems that it can call upon: 1) amino acid transport and uptake, 2) amino acid oxidation and catabolism, 3) protein synthesis, and 4) protein breakdown. Figure 1 gives an overview of the model that is developed in this article.
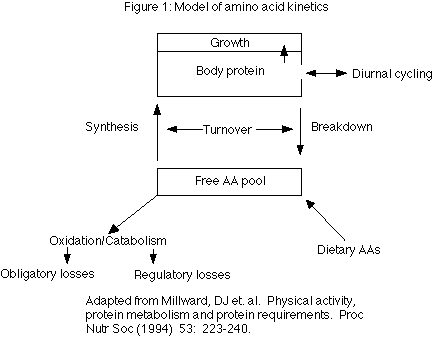
When faced with different stresses (such as high or low protein intakes, or starvation) the body can alter the efficiency of the above pathways in an attempt to maintain body protein stores. Each pathway is discussed in detail below. Since amino acid transport and uptake has been discussed in previous parts of this series, it is not discussed further here. Readers should refer to Part 1 and Part 3 of this series for more details.
Amino acid oxidation and catabolism (2)
The oxidation and catabolism of amino acids were discussed within the context of both exercise and increasing dietary protein in Part 1 of this series but require further discussion here. Oxidation and catabolism of amino acids can occur through two related processes: deamination and transamination.
Both reactions start with the removal of the amino group from the amino acid, leaving a keto acid and ammonium (NH3+). In the case of deamination, NH3+ is formed into waste products (e.g. urea) and disposed of.
The general reaction for deamination looks like this:
Amino acid <————–> keto acid + ammonium
As ammonium is toxic to the body, it must be disposed of. In many tissues, NH3+ combines with glutamate to make glutamine, which then travels to the liver. In the liver, glutamine is broken down to glutamate and ammonium again and the ammonium is formed into urea for excretion.
With transamination, one AA donates its amino group to another compound resulting in the production of a new amino acid and a keto acid. For example, ketoglutarate and pyruvate may bond to ammonium to generate glutamine and alanine respectively. The general reaction for transamination appears below.
Amino acid 1 <—————-> Keto acid 1
/
| NH3+
/
Keto acid 2 <—————-> Amino acid 2
The inclusion of double arrows indicates that these reactions are reversible, meaning that they can proceed in both directions. The formation of glutamine and alanine in the muscle (discussed in Part 3) occurs through transamination and oxidation of other amino acids, especially the branch chain amino acids (BCAAs).
The keto acids (also called the carbon skeleton) formed by de/transamination have a number of fates in the body depending on the metabolic state. They can be used to produce energy directly. Alternately, they may be used for the synthesis of glucose, fatty acids or ketones, or can be reaminated if ammonium is available (2).
Oxidation and catabolism can be further subdivided into obligatory and regulatory losses (Fig 1). Obligatory losses are those that occur as a consequence of normal body functioning and are considered constant regardless of diet or the body’s condition. They will not be discussed further since they cannot be changed.
Regulatory losses are those that occur with changes in diet or exercise. For example, with long-duration aerobic exercise, increased oxidation of certain AAs within the muscle (see Part 3 for details), increases AA losses. As will be discussed below, oxidation of AAs changes in response to changes in protein intake, increasing with higher protein intakes and decreasing with lower protein intakes (3-7).
While many negative statements have been made about AA oxidation and catabolism in recent bodybuilding literature, what is often misunderstood is that the byproducts of AA oxidation may play important roles in metabolism. The increased oxidation of AA is though to play a role in growth and has been termed the ‘anabolic drive’ by protein researchers (8,9). For example, when leucine is oxidized, it produces ketoisocaproate (KIC) which may play a positive role in protein synthesis (10). When KIC is oxidized, beta-hydroxy-methyl butyrate (HMB) is produced which may also play a role in protein synthesis (11), although it’s real-world effects as a supplement have fallen short of expectations. The take-home message is that the oxidation of AAs is not always a negative aspect of AA metabolism.
The free amino acid pool
In the body, there are two sites of AA ‘storage’. The first of these is in tissues such as muscle and liver proteins. The second is the small free AA pool. The free pool exists to provide individual AAs for protein synthesis and oxidation, and it is replenished either by protein breakdown or AAs entering the body from the diet (Figure 1).
The sizes of these two sites are extremely different. In an average 70 kg (154 lb) man, body protein may comprise 10 kg (22 lbs) of AAs. In contrast, the free pool has been estimated to contain only 100 grams of AAs, not including taurine. If taurine is included, the size of the free pool increases to 130 grams (12). There are an additional 5 grams of free AAs circulating in the bloodstream (12). The important point is that the free pool is approximately 1% of the size of the AA stored in tissue.
The free AA pool plays an important role in overall protein metabolism for a number of reasons. Conceptually, the free pool provides the link between dietary protein and body protein (Fig 1) in that both dietary protein and body protein feeds into the free pool. The free pool is also intimately connected with the concept of protein turnover, discussed below.
Perhaps more importantly to bodybuilders, the concentrations of certain AAs in the free pool, notably glutamine, are directly correlated with muscle protein synthesis. Glutamine is the most abundant AA found in the free pool, comprising approximately 60% of the pool and variations in glutamine may have an impact on protein synthesis and breakdown (13,14).
In two similar studies, researchers removed a piece of muscle tissue from a rat and perfused (soaked) it in various nutrients including varying concentrations of glutamine and insulin. They found that the higher the levels of glutamine concentration, the higher the rate of protein synthesis and vice versa (13,14). This result is often cited as evidence for the benefit of glutamine supplementation. Similar arguments have been made for the use of supplemental taurine as a cell-volumizer.
Unfortunately (both for supplement manufacturers and bodybuilders), oral consumption of a nutrient is far different than adding it to a perfused cell. Recall from Part 3 that the profile of amino acids ingested has only a small impact on what amino acids are released into the bloodstream. By the same token, the type and/or amount of protein consumed affects the free pool only slightly.
As a prime example, subjects in one early study were given a huge amount of protein, 3 g protein/kg, sufficient to double normal AA intake. However, blood concentrations of most AAs rose only 30% above normal levels, with concentrations of BCAAs doubling over normal levels (15). This demonstrates how tightly regulated the free pool is, and how ineffective the simple addition of one or another AAs to a supplement will most likely be. Additionally, the concentration of amino acids in the bloodstream differs from that seen within the muscle (16) so even changes in blood AA levels may have no impact on intramuscular AA concentrations.
The body appears to maintain the free AA pool within tight limits (17) and measurements under a variety of conditions find extremely similar values for the free AA pool (16,18). To suggest that bodybuilders can significantly affect the intramuscular AA pool simply by adding several grams of glutamine or a gram of taurine to a protein drink is ludicrous. To quote Furst (16) “The fact that intracellular amino acid pattern is reproducible from one individual to another suggests that the concentration of each individual amino acid in the cell is precisely regulated by the biophysical and biochemical mechanisms.”
However, there may be another,somewhat indirect, mechanism by which oral glutamine could affect growth. As discussed in Part 3, glutamine is involved in acid-base balance, and studies on endurance athletes have shown depressed glutamine levels (19). Additionally, lowered concentrations of glutamine in the free AA pool have been found in trauma and sepsis, and catabolic hormones seem to mediate the loss of glutamine from muscle (16).
Therefore, glutamine’s biggest impact might be to prevent a decrease in the free AA pool (19). However, it is unlikely to increase concentrations above physiologically normal levels. What is needed is for researchers to measure the concentration of glutamine in the free pool before and after weight training as well as before and after glutamine supplementation. This would indicate whether weight training or dietary supplementation do in fact affect the concentrations of glutamine in the free pool.
Protein turnover: the coupling of protein synthesis and protein breakdown
Although protein turnover was mentioned in Part 1 of this series, it’s importance in overall AA metabolism requires that it be discussed further. The body is not static and protein tissue undergoes a constant series of breakdown and resynthesis. It is this constant breaking down and building up of proteins that is referred to as turnover (Figure 1).
The breakdown of tissue provides AAs into the free pool and synthesis occurs by pulling AAs back out of the free pool. It should be noted here that increases in protein synthesis (as occur during growth) are frequently accompanied by increases in protein breakdown, although the reason for this rather wasteful process is unknown (17).
Different tissues have significantly different rates of turnover. For example, while liver proteins may be broken down and resynthesized within a number of hours, skeletal muscle protein may take days to turnover (12,17). Tissues such as ligaments and tendons may take several months to a year to turnover. This has important implications when we look at both short- and long-term protein deprivation and excess below.
It has been estimated that an average-sized individual may break down and resynthesize as much as 300 grams of protein per day under normal dietary circumstances and in a normal physiological state (e.g. not recovering from trauma or illness). Larger and smaller individuals will turnover proportionally more and less total protein per day.
At first glance, protein turnover seems a rather wasteful process for the body to undergo, especially since the net result is more or less maintenance of bodily tissue (since most of the protein broken down is resynthesized again). However, protein turnover may play a very important role for dealing with stressful situations: providing AAs where they are needed.
Reduced protein turnover might compromise the body’s ability to rapidly deal with stressful stimuli (17,20,21). It has been suggested that the enhanced rate of muscle breakdown seen in burn and trauma patients occurs to provide sufficient AAs (especially glutamine and it’s precursors) to sustain the immune system (20). Of course, this occurs at the expense of muscle tissue, explaining the muscle wasting seen in stressful situations.
Protein turnover is mediated by a number of factors. This includes hormonal factors (testosterone, thyroid, insulin, cortisol, GH, glucagon), caloric intake, and AA availability (9). For example testosterone and thyroid can be affected in the longer term by dietary changes (e.g. testosterone levels have been correlated with higher fat intake and levels of thyroid hormones are correlated with both protein and carbohydrate intake). However, on a meal to meal basis the availability of AAs and the concentration of insulin appear to play the major roles in determining protein synthesis and breakdown (22). Both are discussed below. Additionally, the roles of cortisol and weight training are discussed in terms of their role in protein turnover.
AAs and insulin
As stated above, arguably the two biggest modulators of protein turnover are insulin and AAs. Interestingly, they appear to modulate turnover through different mechanisms. Contrary to popular belief and what is written in many textbooks, insulin’s primary role is to decrease protein breakdown, with only a minor role in protein synthesis (23-26) although some studies have shown the opposite to be true (27). In fact, elevating insulin without simultaneously increasing AA availability tends to decrease protein synthesis, due to a decrease in circulating AA concentrations (22,28). In contrast, dietary AAs appear to have their major effect of increasing protein synthesis, with little to no effect on protein breakdown (23,24,29) although not all studies have shown this to be the case (30).
Additionally, studies on glutamine concentrations and protein synthesis (discussed above) have found that protein synthesis is much greater when both glutamine and insulin are present (22). Similar observations have been made for other AAs (22). A recent study found that the provision of only indispensable AAs and glucose (to raise insulin) was sufficient to stimulate protein synthesis (31). Collectively, the above data suggests that optimal results in terms of net protein gains will be had with the combination of AAs and insulin.
The practical implication of this should be clear: to maximally affect protein turnover away from net breakdown and towards net protein synthesis, a constant supply of AAs is required, along with a maintenance of normal insulin levels. This can best be ensured by providing both protein and some carbohydrate at each meal. It should be noted that not much carbohydrate is needed to raise insulin to optimal levels. One recent study found that 30 grams of carbohydrate, along with 13.5 grams of protein was sufficient to increase muscle protein synthesis (31). Even more interesting about this study is that only indispensable AAs were given, suggesting that dispensable AAs are not as critical for protein synthesis.
The branch-chain amino acids (BCAAs)
One class of AAs bears more discussion: the branch-chain amino acids (BCAA). BCAA are unique among AAs in that they are only used for the synthesis of tissue protein but not for the synthesis of other biologically active molecules such as hormones (32). In fact, AA mixtures which lack BCAAs are ineffective at stimulating protein synthesis (33).
While some data suggests that all three BCAAs are important for protein synthesis, leucine is the BCAA that has been the most well studied. Interestingly, while leucine itself seems to modulate protein synthesis, it appears that the oxidative by-product, KIC, inhibits protein breakdown (10). This suggests an important role of AA oxidation in the overall regulation of protein turnover.
The importance of BCAA in determining protein synthesis has led to the frequent suggestion of BCAA supplementation in athletes and there may be some merit to this. Additionally, claims are sometimes made about specific protein sources having higher or lower BCAA concentrations. However, as BCAAs typically make up 50% of the indispensable AAs found in most proteins, deficiencies do not occur in individuals consuming adequate protein (32). Considering the generally high protein intake among bodybuilders, it seems unlikely that the provision of a few grams of BCAAs will have a large impact on growth. Additionally, while endurance training is known to increase the oxidation (and probably the requirement) of BCAAs, it is unknown whether weight training causes a similar result.
Cortisol: friend or enemy?
In general, cortisol is considered the ‘enemy’ to muscle growth in popular bodybuilding literature and this is somewhat true. One of the primary effects of cortisol is to inhibit protein synthesis, so that net breakdown occurs (34). Additionally, cortisol inhibits AA uptake (34). Finally, cortisol appears to preferentially affect muscle protein synthesis. In this regard, cortisol can be thought of as a negative in terms of growth.
However, cortisol plays other critical roles in the body, especially in terms of dealing with inflammation. Individuals using anti-cortisol drugs frequently report joint pain. As discussed above, the breakdown of protein tissue as part of protein turnover is a critical aspect of overall body functioning, as this serves to provide AAs where they are needed during times of stress.
The main regulators of cortisol levels appears to be blood glucose and insulin levels. In general, when insulin and blood glucose are high, cortisol will be low and vice versa. In vitro studies have shown that cortisol does not begin to have it’s major effects on decreasing protein synthesis and increasing protein breakdown for at least 4 hours (22). In addition, the negative effects of cortisol on protein synthesis are reversed with only one hour of refeeding, most likely from the elevation of insulin (22). This strongly suggests that the common practice of eating every few hours is a sound strategy to minimize the effects of cortisol. In fact, one might go even further and get up for a meal in the middle of the night, to prevent cortisol mediated protein breakdown from occurring. Alternately, the consumption of a small meal containing protein, carbohydrate, fat and fiber at bedtime should allow maintenance of blood glucose/insulin levels for at least part of the night.
The effect of weight training
Undoubtably, resistance training affects protein turnover and net gains in body protein. However, it has been shown that weight training affects both protein breakdown and synthesis. Following the performance of a typical bout of weight training, both protein synthesis (35,36) and breakdown (37,38) in the target muscle are increased. However, synthesis is increased moreso than breakdown (37,38) such that there is a net gain in muscle mass.
An interesting study (or group of studies) would be to determine the number of sets which causes the greatest difference in increased protein synthesis and breakdown. It seems reasonable to assume that protein synthesis can only be increased to a certain level, while sets beyond this point serve only to increase protein breakdown more than necessary.
Additionally, there is increased amino acid transport into the muscle following training (38) and it has been shown that the provision of glucose and AAs after training further enhances protein synthesis while inhibiting protein breakdown (38,39). It has been found that protein synthesis remains elevated for at least 36 hours following training (36). This suggests that the most critical time to ensure an excess of AAs is in the time period immediately following a workout. Providing dietary protein and AAs immediately after training and for the next 36 hours should help to maximize protein synthesis and minimize protein breakdown.
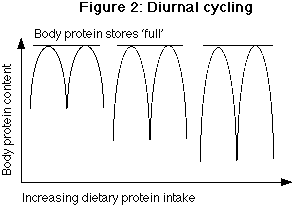
Diurnal cycling
The concept of diurnal cycling is at once critical yet also somewhat irrelevant to the entire concept of AA metabolism. Diurnal cycling refers to the cyclical nature of net protein synthesis during the fed state (when food is being consumed) which is matched by net protein breakdown during the fasted state (when food is not being consumed). This seemingly wasteful process is thought to provide meal derived AAs to tissues more evenly over a 24 hour period (12).
The proteins synthesized after a meal are thought to be labile proteins; that is, proteins which serve mainly as a temporary storage site for protein (12). These labile proteins are broken down during the night, leading to essentially no net gain or losses over a 24 hour period. Diurnal cycling is thought to act as a ‘buffer’ to prevent increases in circulating AAs from occurring after a meal since they are directed into tissue synthesis (41).
Diurnal cycling is sensitive to protein intake. As protein intake goes up, and protein storage during the day increases, there is increased protein breakdown at night (Fig 2). By the same token, when protein intake goes down, there is less protein stored during the day, and less broken down at night. However, whenever there is a change from high to low protein intakes, there is a short lag time before diurnal cycling ‘catches up’. Thus, the more protein an individual eats, the more he or she needs to eat to maintain balance (17,42). The adaptations to varying protein intakes are examined below.
Summary
The above model of AA kinetics, including a discussion of the free AA pool, protein turnover, and diurnal cycling should help readers to understand how protein and AAs are processed within the body, as well as to examine some of the claims currently being made about protein and AA supplements. It will also be useful to understand the adaptations to high- and low-protein intakes discussed below.
Section 9: Adaptations to high- and low- protein intakes
Having developed a model of AA kinetics in the body, we can now examine the adaptations which occur during either high- or low-protein intake. In addition, this allows us to address the recent ideas behind protein cycling. As discussed in the introduction, the body has a number of mechanisms by which it attempts to maintain protein homeostasis. They are discussed here. In addition, we will examine what happens to body protein stores when intake goes from high to low or vice versa.
What is protein cycling?
Before discussing the adaptations to varying protein intakes, a brief overview of the concept of protein cycling needs to be discussed. While far from a new concept, a recent trend in bodybuilding nutrition is the cycling of macronutrients in an attempt to cause specific physiological processes to take place. As an example, many are familiar with the cyclical ketogenic diet (CKD) which alternates periods of low-carbohydrate eating with periods of high-carbohydrate eating.
In the same vein, several authors have suggested that the intake of protein be cycled. The general premise is that chronically high intakes of protein lead to adaptive increases in protein oxidation and breakdown, which is deemed to be negative. By restricting protein for some periods of time (anywhere from 3 days to 1 month has been suggested), these authors suggest that oxidation and breakdown of AAs will be reduced, such that muscle can be gained more quickly when protein is refed at higher levels. One author has even suggested that AA oxidation rates will be permanently ‘reset’ at low levels after a month of low-protein intake. While there are other components to the theory of protein cycling, such as increased GH levels, the above serves as a good overview. The claims made above will be addressed in the following discussion of adaptations to varying protein intakes.
Mechanism of adaptation to changing protein intakes
The body has several potential mechanisms by which it can attempt to maintain protein stores. Arguably the primary method, other than growth, is via alterations in the rate of AA oxidation (17,43). When the intake of an AA is below what is required for maximal growth, oxidation of that AA remains low (43). When human AA intake is in excess of requirements, oxidation is increased. This adaptive response has been demonstrated in several studies on humans, with oxidation increasing or decreasing in response to high- or low-AA intakes (3-7).
In a study involving weight training, subjects received either 1.3 g/kg or 2 g/kg of protein per day (41). In the high protein group, AA oxidation was increased by 150% above normal levels. In addition, while there was no change in protein synthesis or breakdown in the low protein group, the high protein group increased synthesis by 105% and breakdown by 107%. Despite this increase in AA oxidation, there was a significant amount of lean body mass gained, approximately 3 lbs over a 4 week span, further supporting the concept that AA oxidation is not the negative it has been made out to be.
The second mechanism by which the body regulates protein stores is through alterations in protein synthesis and breakdown. In animal models, with as little as 12 hours without food, the rate of muscle protein synthesis falls although this can be reversed within 1 hour of refeeding (22). Although protein breakdown may increase initially (43), there is eventually a decrease in protein breakdown in protein deficient rats (43,44).
In humans, similar results occur: with increased protein intake, there is an overall increase in protein synthesis and breakdown. With decreased protein intake, protein synthesis and breakdown eventually fall so that the body can reattain balance (45,46). Ultimately this reflects an overall decrease in protein turnover (21). In a sense, AAs within the body are being more efficiently reutilized since there is decreased breakdown and oxidation.
A final potential mechanism by which the body can alter protein utilization is through the urea cycle. Recall from above that urea is generated when excesses of ammonium are produced through amino acid oxidation/catabolism. In that AA oxidation increases with increasing intake, we would expect urea production to increase with increased protein intake. Greater amounts of urea nitrogen being lost in the urine with increasing protein intakes. Similarly, decreased urea production is seen with decreasing protein intake (43).
With regards to the concept of protein cycling, the above data is more or less in keeping with the general concept. It is well-established that high-protein intakes (defined as above requirements) increases AA oxidation. As well, a reduction in protein leads over time to a reduction in AA oxidation. The only hole in the theory of protein cycling that we can see so far is represented in the study on weight training discussed above (41). What proponents of protein cycling seem to have forgotten is that only the excess protein is oxidized leaving the amount needed to support growth available to do so. Additionally, even in the face of increased oxidation, the body still maintains a net positive nitrogen balance.
The time course for changes
An important question regarding adaptation to changes in protein intake is how long it takes for the body to adapt. Once again, methodological problems prevent detailed human studies from being done although a few exist.
During protein deprivation in rats, both rates of protein turnover (synthesis and degradation) decrease as the length of time in starvation decreases (47). For example, in one study rats were given an essentially protein-free diet. After 1 day, protein synthesis had dropped by 25-40%. After 3 days, protein breakdown and oxidation had decreased by 30-45%. During refeeding, protein synthesis rose by 30% after 1 day and protein breakdown increased by 60% after day 3. The difference in changes of protein synthesis and breakdown during refeeding probably allows the rat to ‘catch-up’ to normal protein levels (48). In another study of protein deprivation, rats did not lose muscle protein until 9 days of protein deprivation had passed, while liver protein was lost almost immediately (44).
In humans, only a handful of studies have been done. Studies of complete starvation have found an increase in nitrogen losses (indicative of increased protein breakdown) during the first week or so followed by a decrease over the next several weeks (49). In another study, subjects were placed on varying levels of protein intake and a variety of measures were made after 7 days. With low protein intakes, protein breakdown dropped 24% within 7 days indicating the rapidity with which the body can adapt (46). In a third study, a reduction in protein breakdown was seen after only two low-protein meals (50). Overall, it appears that rates of protein and breakdown and synthesis can vary depending on protein intake as part of the overall adaptations.
In one detailed study, subjects were placed on either a low (96 gram/day) or high (260 gram/day) protein diet, which was maintained for 50 days (51). At that time, the groups switched from either high- to low-protein or vice versa. While only nitrogen balance was measured (i.e. no measurement of protein synthesis, breakdown or oxidation were performed), the results are interesting and applicable to the idea of protein cycling (discussed below). After the switch from low to high protein intakes, there was a large positive nitrogen balance persisting for 9-12 days before adaptation had occurred. by the same token, when subjects switched from high to low protein intakes, there was a large negative nitrogen balance which lasted for about 9-12 days before the body adapted. This has important implications for protein cycling, discussed below.
With regards to protein cycling, the above data supports the idea of short (3-12 day cycles of alternating protein intake) far more than it does the idea of a full month of low-protein. It appears that the majority of adaptations has taken place in a fairly short time period. Longer periods of low-protein would only serve to further deplete protein stores. Additionally, there is no evidence in humans to support the idea that AA oxidation remains low permanently. In fact, this would run quite contrary to the body’s goal of maintaining homeostasis.
Where does the protein come from/go to?
Perhaps the most important consideration when examining the adaptations to different protein intakes is where the protein is being lost (during protein deprivation) and gained (during protein refeeding). Once again, we must look at animal studies although they provide an incomplete picture for humans.
Recall from above that tissues vary in their rates of turnover. Liver proteins may be broken down and replaced completely within several hours, while muscle protein may take several days. Tendons and ligaments may take months to a year to turnover. Because of these differences, we would expect there to be differences in the site of protein synthesis and breakdown. Due to their short turnover time (several minutes), liver proteins are thought to be the site of short-term synthesis (after meals) and breakdown (during fasting) (1,22,34).
Unfortunately, it is methodologically difficult to determine where protein is being lost in humans so we must look at animal models. It should be noted that there are significant differences between animal and human protein metabolism so extrapolation should be made with care. During starvation in rats, the first proteins to be lost are from the liver, with 25-40% of liver protein being lost after 48 hours (34,47). Decreases in the size of other organs such as the heart and brain has also been noted. Muscle protein is not lost until several days later. Considering differences in the rate of protein turnover (see above), it makes sense that proteins with the fastest rates of turnover would be lost first. Readers should note that 48 hours of starvation in the rat corresponds to longer periods of starvation in man.
By corollary, during refeeding, we would expect that the first proteins to be repleted are liver proteins, with muscle protein being rebuilt afterwards. So while it has been suggested that the low-protein days will cause the loss of liver proteins while the high-protein days will cause the gain of mainly muscle proteins, this seems a highly illogical path for the body to take as it would allow the progressive depletion of organ proteins with the progressive growth of muscle protein. This would most likely eventually result in the death of the organism.
In addition, the studies cited above demonstrate perhaps the biggest problem with the whole concept of protein cycling, as the body’s protein stores are repleted (sometimes referred to as ‘catch-up’ growth), rates of oxidation, synthesis and breakdown return to the levels they were at prior to the low-protein phase. So not only does the body appear to first replete those proteins which were first lost (liver and other organ proteins), but by the time those proteins are repleted, the body has readapted to the current level of protein intake. In all likelihood, the net result of protein cycling will be no change in total body protein stores. This should be contrasted to simply keeping protein intake at appropriate levels (at or slightly less than 1 g/lb as discussed in Part 1) to allow gains to occur at their normal rate.
Summary
There are a number of mechanisms by which the body can adapt to increasing and decreasing protein intakes. Arguably the most important is rates of oxidation, which can increase or decrease rapidly to compensate for increasing and decreasing protein intakes. In addition, rates of protein synthesis and breakdown can be altered, with both typically decreasing with lowered protein intakes, and increasing with raised protein intakes. Finally, the amount of urea produced, which is related to AA oxidation, may be altered.
Although comprehensive data is lacking, it appears that the major adaptations to altered protein intakes take place fairly rapidly, within a number of days. In rats, this may be 3-7 days, in humans 9-12 days or slightly longer.
While more research is needed, it appears the the first proteins lost during protein deprivation are liver and other organ proteins. By the same token, during protein refeeding (or simply high protein feeding) it appears that liver proteins are the first to be synthesized.
With regards to the concept of protein cycling, while the general idea is somewhat logical, in that decreasing protein intake can cause a transient decrease in oxidation and turnover, there is little indication that there will be a net gain in body protein when protein intake is increased again. In the same way that liver proteins are the first lost, they will likely be the first regained. And by the time liver proteins have been rebuilt, rates of oxidation and turnover will have returned to normal, leaving the individual with no net gains.
References
1. Waterlow, JC. Where do we go from here? J Nutr (1994) 124:1524S-1528S.
2. Advanced Nutrition and Human Metabolism, 2nd ed. James L. Groff, Sareen S. Gropper, Sara M. Hunt. West Publishing Company, 1995.
3. Meguid, MM et. al. Leucine kinetics at graded leucine intakes in young men. Am J Clin Nutr (1986) 43: 770-780.
4. Meguid, MM et. al. Valine kinetics at graded valine kinetics in young men. Am J Clin Nutr (1986) 43: 781-786.
5. Meredith, CN et. al. Lysine kinetics at graded lysine intakes in young men. Am J Clin Nutr (1986) 43: 787-794.
6. Zhao, Xi-he et. al. Threonine kinetics at graded threonine intakes in young men. Am J Clin Nutr (1986) 43: 795-802.
7. Motil, KJ et. al. Whole body leucine and lysine metabolism: Response to dietary protein intake in young men. Am J Physiol (1981) 240: E712-E721.
8. Millward, DJ. The endocrine response to dietary protein: the anabolic drive on growth. In Milk Proteins: Nutritional, clinical, functional and technological aspects. C.A. Barth, E. Schlimme (Eds.) Springer Verlag, 1989.
9. Millward, DJ and Rivers, JPW. The need for indispensable amino acids: the concept of the anabolic drive. Diabetes/Metabolism Rev (1989) 5:191-211.
10. Tischler, ME et. al. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem (1982) 257: 1613-1621.
11. Nissen, S et. al. Effect of leucine metabolite B-hydroxy-B-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol (1996) 81: 2095-2104.
12. Wagenmakers, AJ. Protein and amino acid metabolism in human muscle. Skeletal Muscle Metabolism in Exercise and Diabetes. ed. Richter et. al. Plenum Press: New York, 1998.
13. MacLennan, PA et. al. A positive relationship between protein synthetic rate and intracellular glutamine concentration in perfused rat skeletal muscle. FEBS Lett (1987) 215: 187-191.
14. MacLennan, PA et. al. Inhibition of protein breakdown by glutamine in perfused rat skeletal muscle. FEBS Lett (1988) 237: 133-136.
15. Wahren, J et. al. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest (1976) 57: 990-995.
16. Furst, P. Intracellular muscle free amino acids – their measurement and function. Proc Nutr Soc (1983) 42: 451-462.
17. Waterlow, JC. Protein turnover with special reference to man. Q J Exp Phys (1984) 69: 409-438.
18. Scriver, CR et. al. Normal plasma amino acid value in adults: The influence of some common physiological variables. Metabolism (1985) 34: 868-873.
19. Antonio J and Street C. Glutamine: A potentially useful supplement for athletes. Can J Appl Physiol (1999) 24: 1-14.
20. Young, V. 1987 McCollum lecture. Kinetics of human amino acid metabolism: nutritional implications and some lessons. Am J Clin Nutr (1987) 46: 709-725.
21. Waterlow, JC. Metabolic adaptations to low intakes of energy and protein. Ann Rev Nutr (1986) 6: 495-526.
22. McNurlan, MA and Garlick, PJ. Influence of nutrient intake on protein turnover. Diabetes/Metabolism Rev (1989) 5: 165-189.
23. Castellino, P et. al. Effect of insulin and plasma amino acid concentrations on leucine metabolism in man. Role of substrate availability on estimates of whole body protein synthesis. J Clin Invest (1987) 80: 1784-1793.
24. Tessari, P et. al. Differential effects of hyperinsulinemia and hyperaminoacidemia on leucine-carbon metabolism in vivo. Evidence for distinct mechanisms in regulation of net amino acid deposition. J Clin Invest (1987) 79: 1062-1069.
25. Heslin, MJ et. al. Effect of hyperinsulinemia on whole body and skeletal muscle leucine carbon kinetics in humans. Am J Physiol (1992) 262: E911-E918.
26. Gelfland, RA and Barrett EJ. Effect of physiologic hyperinsulemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest (1987) 80: 1-6.
27. Biolo, G et. al. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest (1995) 95: 811-819.
28. Frexes-Steed M et. al. Role of leucine and other amino acids in regulating protein metabolism in vivo. Am J Physiol (1992) 262: E925-935.
29. Svanberg, E et. al. Effects of amino acids on synthesis and degradation of skeletal muscle proteins in humans. Am J Physiol (1996) 340: E718-E724.
30. Giordano, M et. al. Differential responsiveness of protein synthesis and degradation to amino acid availabiitiy in humans. Diabetes (1996) 45: 393-399.
31. Tipton, KD et. al. Nonessential amiono acids are not necessary to stimulate net muscle protien synthesis in healthy volunteers. J Nutr Biochem (1999) 10: 89-95.
32. 141. Harper, AE et. al. Branched-chain amino acid metabolism. Ann Rev Nutr (1984) 4:405-454.
33. May, ME and Buse, MG. Effects of branch-chain amino acids on protein turnover. Diabetes/Metabolism Rev (1989) 5: 227-245.
34. Kettelhut, IC et. al. Endocrine regulation of protein breakdown in skeletal muscle. Diabetes/Metabolism Rev (1988) 4: 751-772.
35. Chesley, A et. al. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol (1992) 73: 1383-1388.
36. MacDougall, JD et. al. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol (1995) 20: 480-486.
37. Phillips, SM et. al. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol (1997) 273: E99-E107, 1997.
38. Biolo, G et. al. Increased rates of protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol (1995) 268: E514-E20.
39. Biolo, G et. al. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol (1997) 273: E122-E129.
40. Roy, BD et. al. Effect of glucose supplement timing on protein metabolism after resistance training. J Appl Physiol (1997) 82: 1882-1888.
41. 114. Fern, EB et. al. Effects of exaggerated amino acid and protein supply in man. Experientia (1991) 47: 168-172.
42. Millward, DJ et. al. Physical activity, protein metabolism and protein requirements. Proc Nutr Soc (1994) 53: 223-240.
43. Young, VR and Marchini, JS. Mechanisms and nutritional significance of metabolic responses to altered intakes of protein and amino acids, with reference to nutritional adaptation in humans. Am J Clin Nutr (1990) 51: 270-289.
44. Garlick, PJ et. al. The effect of protein deprivation and starvation on the rate of protein synthesis in tissues of the rat. Biochmica et Biophysica Acta (1975) 414: 71-84.
45. Hoerr, RA et. al. Effect of protein restriction and acute refeeding on leucine and lysine kinetics in young men. Am J Physiol (1993) 264: E567-E575.
46. Yang, RD et. al. Response of alanine metabolism in humans to manipulations of dietary protein and energy intakes. Am J Physiol (1986) 250: E39-E46.
47. Mortimore, GE and Poso, AR. Intracellular protein catabolism and its control during nutrient deprivation and supply. Ann Rev Nutr (1987) 7:539-564.
48. Tawa, NE and Goldberg, AL. Supression of muscle protein turnover and amino acid degradation by dietary protein deficiency. Am J Physiol (1992) 263: E317-E325.
49. Cahill G. Starvation in man. N Engl J Med (1970) 282: 668-675.
50. 175. Taveroff, A et. al. Mechanism governing short-term fed-state adaptations to dietary protein restriction. Metabolism (1994) 43: 320-327.
51. 172. Oddoye, EA and Margen, S. Nitrogen balance studies in humans: long-term effect of high nitrogen intake on nitrogen accretion. J Nutr (1979) 109: 363-377.
About the author
Lyle McDonald+ is the author of the Ketogenic Diet as well as the Rapid Fat Loss Handbook and the Guide to Flexible Dieting. He has been interested in all aspects of human performance physiology since becoming involved in competitive sports as a teenager. Pursuing a degree in Physiological Sciences from UCLA, he has devoted nearly 20 years of his life to studying human physiology and the science, art and practice of human performance, muscle gain, fat loss and body recomposition.
Leave a Reply
You must be logged in to post a comment.