
I. Preamble
Many of you may have already come across my original Science of Trenbolone “article” that has been circulating bodybuilding boards for many years. I use the term “article” quite loosely though, as I never realized at the time that it would become so widely shared. The original intent of that “article” was simply to make a post on a private bodybuilding board that I spent about ten minutes putting together. It was designed to be a quick and dirty reference guide to help answer many questions that were being asked about regularly. So, it goes without saying that whenever I see it being shared, I get a pang of embarrassment as it has my name attached to it and has grown to become a bit of an authority piece on trenbolone.
This will be the article that should have been written then, and the one that I encourage folks to rely upon moving forward.
II. Introduction
Trenbolone is a hormone that has an almost mythical reputation within bodybuilding circles. Because there is very limited data on humans, we often must rely upon anecdotes when trying to form hypotheses. As can be seen on just about any bodybuilding board, experiences with trenbolone vary widely – with some absolutely worshipping the compound and others either advising extreme caution or telling folks to avoid it at all costs. Despite this large divide in opinion, there is no disputing its popularity as numerous surveys over the years have demonstrated it to be one of the most frequently used anabolic compounds, with anywhere between 20-25% of enhanced bodybuilders reporting they’ve used it within the last twelve months [1, 2, 3].
My goal with this article will be to use what information is available to try and form some more solid conclusions with regard to how the compound works. At the same time I hope to help dispel some myths that are still being propagated far too often.
As I mentioned, there are only one or two controlled human trials that I’m aware of so the vast majority of cited material is going to come from either animal studies or in vitro analysis. The question that must be asked is can we take this data and apply it to bodybuilders with any semblance of accuracy? Personally, I feel there are going to be some very concrete bits that are universally applicable to humans and then there are some that may require disclaimers. I will try and do my best to point these out as the article goes on.
III. Basics of Trenbolone
Trenbolone is a selective androgen receptor modulator (SARM) not designed for human use [4]. Despite this designation, it continues to be heavily used by bodybuilders for muscle growth, fat reduction, and body composition purposes [5–6]. SARMs are modified analogues of male sex hormones normally exhibiting favorable anabolic activity while simultaneously having moderate-to-minimal androgenic activity in vivo as compared to native androgens [7, 8, 9]. They are under development by many pharmaceutical firms in an attempt to create alternative means to treat conditions such as hypogonadism, as well as other muscle and bone wasting states. In essence, the goal is to recreate the positive aspects of supraphysiological doses of testosterone while simultaneously removing the risk for adverse events that tend to occur when using these high doses [10].
Most all SARMs start off life as a testosterone molecule. The chemical structure of the testosterone molecule is then traditionally modified in one of three ways [11–12]:
- Esterification at the 17β-hydroxyl group which increases hydrophobicity or the likelihood of a molecule to be repelled from a mass of water
- Alkylation at the 7α-position which reduces 5α-reductase binding affinity
- Strategic modification of the C1, C2, C9, C11, or C19 carbons to achieve a wide range of therapeutic effects
Trenbolone is a C19 norandrogen (19-nor), derived from nandrolone (nortestosterone). Removal of the methyl group at position 19 of the steroid backbone significantly reduces the susceptibility of 19-nor androgens to aromatize as well as undergo 5α-reduction [4]. We’ll be getting deeper into the underlying mechanisms later but, for now, just understand that subtle modifications to the cholesterol backbone of the testosterone molecule can directly translate into significant changes to the behavior of the new SARM molecule. Some of these changes can include the SARM’s binding affinity for the androgen receptor as well as its binding affinity with numerous enzymes capable of converting SARMs to other steroids [13]. Trenbolone has SARM-like properties in that it has significant less affinity for testosterone’s downstream pathways. We’ll talk much more about this later.
IV. History of Trenbolone
The enormous anabolic potential of trenbolone, as well as its analogs, was reported as far back as the 1960s. There was also an oral version created (methyl-trenbolone), however it has never been marketed as an anabolic agent due to its extreme liver toxicity – causing intrahepatic cholestasis at orally administered amounts as small as 1mg/day [14].
It has never been approved for human use and trenbolone is now primarily used as a growth promoting agent in livestock [15–16]. It is used natively as well as in combination with estradiol (E2) [17]. The use of implants containing the combination of androgenic and estrogenic steroids was approved by the FDA in 1992 [18] and now approximately 90% of beef cattle in the United States are being treated with a growth-promoting mix of estrogens, androgens, and/or progestins [19]. Implants are big business with up to 20 million cattle per year implanted with trenbolone and annual revenues likely exceeding a billion dollars [20].
Despite the FDA’s approval, there are still safety concerns as trenbolone acetate (TBA) and its metabolites have been identified as potential endocrine disrupting chemicals (EDCs). EDCs are exogenous molecules that can mimic or inhibit the action of sexual hormone receptors such as estrogen, androgen and thyroid hormone receptors. These EDCs can also disrupt the synthesis, movement, metabolism, and secretion of naturally occurring hormones which may lead to serious issues down the line including obesity, diabetes and even cancer [21–22].
Because of the potential severity of EDCs, the last two decades has seen higher international attention on environmental exposure, and the effects of EDCs in humans and wildlife [23–24]. As mentioned just a moment ago, TBA and its metabolites have been identified as EDCs through many studies, can be widespread in agricultural environments, and are associated with reproductive toxicity [25, 26, 27]. And it only takes exposure in very low concentrations to cause potential problems, as has been demonstrated in animals like fish with skewed sex ratios and decreased fertility [28].
It will also be important to be able to distinguish the various types of TBA implants, as many of the studies we’ll be reviewing later use different types on their subject animals. What follows is a list of common implant types used in the United States, along with their hormonal concentrations:
- Revalor-XS (200mg TBA / 40mg E2)
- Revalor-200 (200mg TBA / 20mg E2)
- Revalor-H (140mg TBA / 14mg E2)
- Revalor-S (120mg TBA / 24mg E2)
- Revalor-IS (80mg TBA / 16mg E2)
- Revalor-IH (80mg TBA / 8mg E2)
- Revalor-G (40mg TBA / 8mg E2)
- Synovex PLUS (200mg TBA / 28mg E2)
- Synovex-C (100mg Progesterone / 10mg E2)
- Synovex-ONE Grass (150mg TBA / 15mg E2)
- Synovex-S (200mg Progesterone / 20mg E2)
- Synovex-H (200mg Testosterone / 20mg E2)
V. Metabolism and Physiology
We made passing mentions to TBA’s metabolites, so let’s spend some time getting into the particulars. It is worth restating now that the vast majority of our knowledge on trenbolone’s in vivo metabolism comes from livestock and rodents [29, 30, 31]. It is also paramount to understand that there are marked differences in the amounts of various metabolites observed in rat and cow models, the two most heavily studied mammals [32]. We will circle back around to this in a moment after first going over some more of the basics.
The chemical alias of TBA is 17ß-hydroxy-estra-4,9,11-trien-3-one-17-acetate, sometimes shortened to 17β-TBOH-acetate. Following an intramuscular injection, it is rapidly hydrolyzed to the biologically active metabolite known as 17β-hydroxy-estra-4,9,11-trien-3-one, or 17β-TBOH [33]. From there it is further broken down into metabolites including glucuronides (e.g. trendione/TBO) and five other polar hydroxylated metabolites [34]. A general flow of the process can be summarized as follows:
- 17ß-hydroxy-estra-4,9,11-trien-3-one-17-acetate / 17β-TBOH-acetate (trenbolone acetate)
- 17ß-hydroxy-estra-4,9,11-trien-3-one / 17β-TBOH
- Estra-4,9,11-triene-3,17-dione / TBO (trendione)
- 17a-hydroxy-estra-4,9,11-trien-3-one / 17α-TBOH (epitrenbolone)
17β-TBOH has a greater affinity for the AR than any of its primary metabolites suggesting that the biotransformation of trenbolone reduces the biological activity of the steroid [25–26,34]. To put this into perspective, in one study the high affinity of 17β-TBOH to the human androgen receptor and the bovine progesterone receptor was reduced after being metabolized into 17α-TBOH and TBO to less than 1/24th of the original compound [35]. This behavior is in stark contrast to testosterone whose conversion to DHT and estrogen leads to more potent compounds as it relates to receptor binding affinity [36–37]. However, TBA’s behavior is similar in nature to other 19-nor behavior (such as nandrolone), whose AR affinity decreases when it is 5-alpha reduced [38].
As mentioned earlier, there is some variation in the metabolism of 17β-TBOH among mammals as the primary metabolites are 17ß-hydroxy-estra-4,9,11-trien-3-one and Estra-4,9,11-triene-3,17-dione together with their 16α and 16ß-hydroxylated in the rat. In the cow, these metabolites were negligible and 17α-TBOH was the major product together with small amounts of 16α and 16ß-hydroxy-17α-TBOH [29–30]. A detailed chart comparing the differences between animals follows:
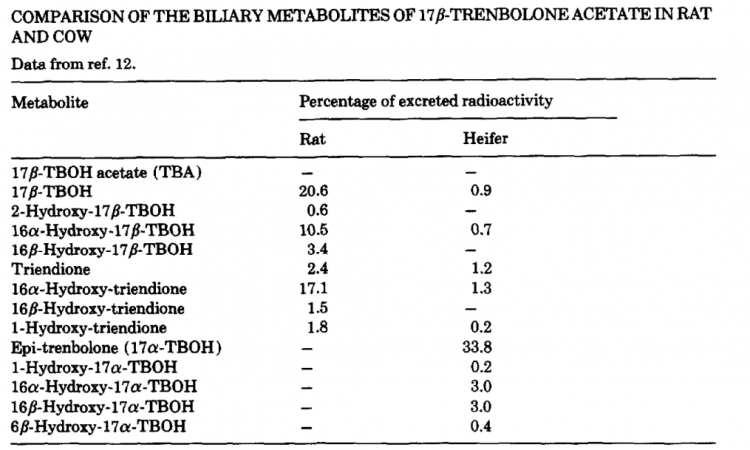
Fortunately for us, there has been a human trial, which I affectionately refer to as the “human hamburger trial”, that helps elucidate how humans metabolize trenbolone – at least after being orally ingested [34]. The trial was designed to investigate the impacts of ingesting tainted food and therefore the research team injected 17β-TBOH into a 5g piece of fried hamburger, at a dose of 0.04 mg/kg of body weight. After a single oral consumption, 63% of the administered dose was excreted via urine by the 72 hour mark; at 24 hours 50% of the administered dose was seen in urine samples.
The results also revealed that, in humans, ingested 17β-TBOH is primarily excreted intact as 17β-TBOH, as epitrenbolone (17α-TBOH), or as trendione (TBO) – with the vast majority being in 17α-TBOH form. In this respect, the biotransformation of 17ß-TBOH in humans more closely resembles that of cows than rodents. In addition, several yet to be identified polar metabolites of 17β-TBOH have been detected in human urine, albeit in a much lower concentration than those metabolites previously mentioned above [39].
17β-TBOH has a low oral bioavailability because it is not methylated at the 17α position. Results from two Hershberger assays demonstrate that trenbolone was about 80–100 fold less effective via the oral route than via injection [25]. Despite this, TBA and 17β-TBOH have still been shown to disrupt the reproductive system of humans, pigs, mice, rats, and other mammalian species at relatively low dosage levels when administered orally.
In the next part of this article series, we’ll explore trenbolone’s impacts on various anabolic pathways as well as metabolic health markers. We’ll also look at how trenbolone impacts endogenous hormone production and potentially even begin our deep-dive into its effects on anabolism and hypertrophy.
References
- Perry PJ, Lund BC, Deninger MJ, Kutscher EC, Schneider J. Anabolic steroid use in weightlifters and bodybuilders: an internet survey of drug utilization. Clin J Sport Med. 2005 Sep;15(5):326-30.
- Parkinson AB, Evans NA. Anabolic androgenic steroids: a survey of 500 users. Med Sci Sports Exerc. 2006 Apr;38(4):644-51.
- Ip EJ, Barnett MJ, Tenerowicz MJ, Perry PJ. The Anabolic 500 survey: characteristics of male users versus nonusers of anabolic-androgenic steroids for strength training. Pharmacotherapy. 2011 Aug;31(8):757-66.
- Donner DG, Beck BR, Bulmer AC, Lam AK, Du Toit EF. Improvements in body composition, cardiometabolic risk factors and insulin sensitivity with trenbolone in normogonadic rats. Steroids. 2015 Feb;94:60-9.
- Daniels JM, van Westerloo DJ, de Hon OM, Frissen PH. [Rhabdomyolysis in a bodybuilder using steroids]. Ned Tijdschr Geneeskd. 2006 May 13;150(19):1077-80. Dutch.
- Geraci MJ, Cole M, Davis P. New onset diabetes associated with bovine growth hormone and testosterone abuse in a young body builder. Hum Exp Toxicol. 2011 Dec;30(12):2007-12.
- Omwancha J, Brown TR. Selective androgen receptor modulators: in pursuit of tissue-selective androgens. Curr Opin Investig Drugs. 2006 Oct;7(10):873-81. Review.
- Gao W, Dalton JT. Expanding the therapeutic use of androgens via selective androgen receptor modulators (SARMs). Drug Discov Today. 2007 Mar;12(5-6):241-8. Review.
- Thevis M, Schänzer W. Synthetic anabolic agents: steroids and nonsteroidal selective androgen receptor modulators. Handb Exp Pharmacol. 2010;(195):99-126.
- Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, Bhasin S. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005 Nov;60(11):1451-7.
- Kicman AT. Pharmacology of anabolic steroids. British Journal of Pharmacology. 2008;154(3):502-521.
- Haendler B, Cleve A. Recent developments in antiandrogens and selective androgen receptor modulators. Mol Cell Endocrinol. 2012 Apr 16;352(1-2):79-91.
- Fragkaki AG, Angelis YS, Koupparis M, Tsantili-Kakoulidou A, Kokotos G, Georgakopoulos C. Structural characteristics of anabolic androgenic steroids contributing to binding to the androgen receptor and to their anabolic and androgenic activities. Applied modifications in the steroidal structure. Steroids. 2009 Feb;74(2):172-97.
- Krüskemper HL, Noell G. Liver toxicity of a new anabolic agent: methyltrienolone (17-alpha-methyl-4,9,11-estratriene-17 beta-ol-3-one). Steroids. 1966 Jul;8(1):13-24.
- Hunt DW, Henricks DM, Skelley GC, Grimes LW. Use of trenbolone acetate and estradiol in intact and castrate male cattle: effects on growth, serum hormones, and carcass characteristics. J Anim Sci. 1991 Jun;69(6):2452-62.
- Chung KY, Johnson BJ. Application of cellular mechanisms to growth and development of food producing animals. J Anim Sci. 2008 Apr;86(14 Suppl):E226-35.Epub 2007 Oct 26. Review.
- Metzler M, Pfeiffer E. Genotoxic potential of xenobiotic growth promoters and their metabolites. APMIS. 2001 Feb;109(2):89-95. Review.
- Chung KY, Baxa TJ, Parr SL, Luqué LD, Johnson BJ. Administration of estradiol, trenbolone acetate, and trenbolone acetate/estradiol implants alters adipogenic and myogenic gene expression in bovine skeletal muscle. J Anim Sci. 2012 May;90(5):1421-7.
- Balter M. Scientific cross-claims fly in continuing beef war. Science. 1999 May 28;284(5419):1453, 1455.
- Lawrence JD, Ibarburu MA. Proceedings of the NCCC-134 Conference on Applied Commodity Price Analysis, Forecasting, and Market Risk Management; 16 and 17 April 2007; Chicago.
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009 Jun;30(4):293-342. Review.
- Rachoń D. Endocrine disrupting chemicals (EDCs) and female cancer: Informing the patients. Rev Endocr Metab Disord. 2015 Dec;16(4):359-64.
- Birnbaum LS. State of the science of endocrine disruptors. Environ Health Perspect. 2013 Apr;121(4):A107.
- Baynes RE, Dedonder K, Kissell L, Mzyk D, Marmulak T, Smith G, Tell L, Gehring R, Davis J, Riviere JE. Health concerns and management of select veterinary drug residues. Food Chem Toxicol. 2016 Feb;88:112-22.
- Wilson VS, Lambright C, Ostby J, Gray LE Jr. In vitro and in vivo effects of 17beta-trenbolone: a feedlot effluent contaminant. Toxicol Sci. 2002 Dec;70(2):202-11.
- Durhan EJ, Lambright CS, Makynen EA, Lazorchak J, Hartig PC, Wilson VS, Gray LE, Ankley GT. Identification of metabolites of trenbolone acetate in androgenic runoff from a beef feedlot. Environ Health Perspect. 2006 Apr;114 Suppl 1:65-8.
- Gall HE, Sassman SA, Lee LS, Jafvert CT. Hormone discharges from a midwest tile-drained agroecosystem receiving animal wastes. Environ Sci Technol. 2011 Oct 15;45(20):8755-64.
- Jensen KM, Makynen EA, Kahl MD, Ankley GT. Effects of the feedlot contaminant 17alpha-trenbolone on reproductive endocrinology of the fathead minnow. Environ Sci Technol. 2006 May 1;40(9):3112-7.
- Pottier J, Busigny M, Grandadam JA. Plasma kinetics, excretion in mild and tissue levels in the cow following implantation of trenbolone acetate. J Anim Sci. 1975 Sep;41(3):962-8.
- Pottier J, Cousty C, Heitzman RJ, Reynolds IP. Differences in the biotransformation of a 17 beta-hydroxylated steroid, trenbolone acetate, in rat and cow. Xenobiotica. 1981 Jul;11(7):489-500.
- Evrard P, Maghuin-Rogister G, Rico AG. Fate and residues of trenbolone acetate in edible tissues from sheep amd calves implanted with tritium-labeled trenbolone acetate. J Anim Sci. 1989 Jun;67(6):1489-96
- Metzler M. Metabolism of some anabolic agents: toxicological and analytical aspects. J Chromatogr. 1989 Apr 7;489(1):11-21.
- Dorts J, Richter CA, Wright-Osment MK, Ellersieck MR, Carter BJ, Tillitt DE. The genomic transcriptional response of female fathead minnows (Pimephales promelas) to an acute exposure to the androgen, 17beta-trenbolone. Aquat Toxicol.2009 Jan 18;91(1):44-53.
- Spranger B, Metzler M. Disposition of 17 beta-trenbolone in humans. J Chromatogr. 1991 Apr 5;564(2):485-92.
- Bauer ER, Daxenberger A, Petri T, Sauerwein H, Meyer HH. Characterisation of the affinity of different anabolics and synthetic hormones to the human androgen receptor, human sex hormone binding globulin and to the bovine progestin receptor. APMIS. 2000 Dec;108(12):838-46.
- Wilson JD. The role of 5alpha-reduction in steroid hormone physiology. Reprod Fertil Dev. 2001;13(7-8):673-8. Review.
- Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev. 2009 Jun;30(4):343-75.
- Sundaram K, Kumar N, Monder C, Bardin CW. Different patterns of metabolism determine the relative anabolic activity of 19-norandrogens. J Steroid Biochem Mol Biol. 1995 Jun;53(1-6):253-7.
- Yarrow JF, McCoy SC, Borst SE. Tissue selectivity and potential clinical applications of trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): A potent anabolic steroid with reduced androgenic and estrogenic activity. Steroids. 2010 Jun;75(6):377-89.
About the author
Chester “Chest” Rockwell is head coach of TeamStackingPlates (TSP). For more information on the team, coaching inquiries, as well as more articles, please visit the team website.
