
Estimated reading time: 1 minute
Table of contents
Introduction
Testosterone (T; 17β-hydroxy-androst-4-ene-3-one), a member of the androst-4-ene-3-one class of anabolic-androgenic steroids (AAS), is the aromatizable and 5α-amplified primary endogenous sex hormone in men. Figure 1. [1]. Besides its being uniquely bioidentical, self-evidently serving as the lynchpin of the most integral aspects of male physiology, its exceptionalism derives specifically from its indispensable support of male sexual function, modulation of glucocorticoids to regulate insulin sensitivity, and muscle anabolic functions via WISP-2 (WNT1-inducible-signaling pathway protein 2; CCN5). For practical bodybuilding use, T is so basically essential that it is virtually ubiquitous in AAS cycle design due to its synergistic (greater than additive) combination with the universe of AAS with respect to skeletal muscle anabolism, fat mass catabolism, and support of male sexual function that characterize its intuitively harm reductive properties – the genesis for the maxim that, “Test is Best.”
Research and development of AAS in the 1960s and 1970s was fervently aimed at developing a pure anabolic, devoid of the side effects associated with aromatization and 5α-reduction. The pharmaceutical industry was practically obsessed with finding the Holy Grail for treating conditions such as cachexia and burn injury without these side effects. By the 1990s, public policy efforts were a response to the advent of high profile sports cheats and widespread doping that resulted in criminalization and the promulgation of the WADA Code. The mid-2000s was a positive inflection point, though brief, for the revitalization of AAS research and development, ushering in the prohormone and designer steroid era (e.g., THG, 4-diols, 19-nor-diols, 17α-methyl analogues, 1-Testo). Again, criminalization was the public policy response that followed until the 2010s experienced a revitalization of… basic testosterone!
For good reason, modern TRT uses the classical androgen, basic T, to great effect for treatment of hypogonadism and to reverse the age-related decrements to libido, muscle size and strength, and metabolic health. While T’s popularity results from its basic efficacy to the user and acknowledgement that its sum is greater than its parts by the practitioner, this author hopes to, in the spirit of reductionism, give insight into some of the features of these parts that make up the whole of T.
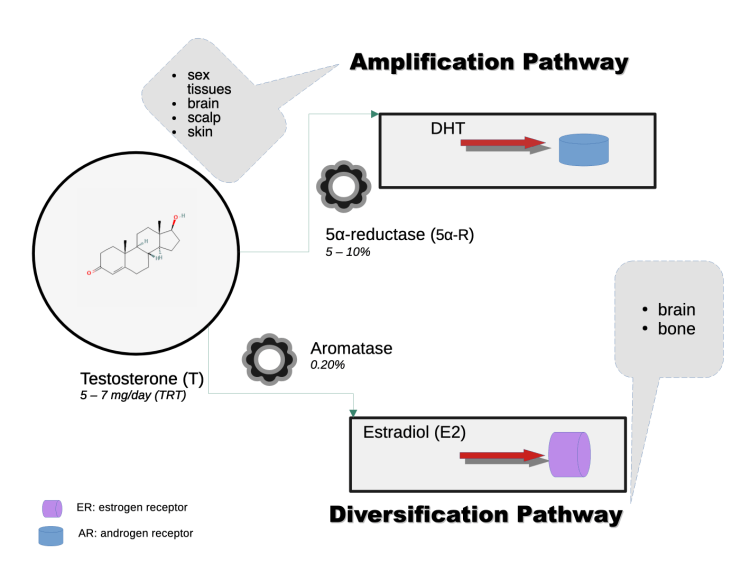
Testosterone stands alone among the universe of commercially available AAS as the sole agent that is both amplified to a more potent androgen (5α-dihydrotestosterone; DHT) by the 5α-reductase enzyme and extensively metabolized by the aromatase enzyme to the aromatic product estradiol (E2). This has several important implications for its particular features and effects in man.
This is the fifth installment in an eleven-part series on the unique characteristics of various AAS.
Sexual Function
T via its extensive aromatization to E2 and 5α-reduction to the most potent endogenous androgen, DHT, in gonadal and CNS tissues, and subsequent 3-keto-reduction of DHT to 5α-androstane-3α,17β-diol (3α-diol) via 3α-HSD, complementarily modulate sexual function in a pluripotent manner vis-à-vis pregnanolone and allopregnenolone, and direct effects – acting in a coordinated lockstep – on hypothalamic and limbic brain regions to stimulate sexual arousal, attention, and behavior. [2]. [3]. [4]. [5]. T’s unique Amplification Pathway (Figure 1) via its reduction by the 5α-reductase enzyme that is ubiquitous in cells of the male sex tissues (e.g., epididymis, seminal vesicles, prostate) and CNS (i.e., brain, spinal cord), produce the most potent endogenous androgen, DHT, at levels up to hundredfold-times greater than in blood. These male sex tissues function interdependently to maintain sperm quality. T’s Diversification Pathway (Figure 1) via its aromatization by the aromatase enzyme that is present in various tissues the body’s tissues, including testes, brain, and adipose tissue, produce the most potent endogenous estrogen, E2. [6]. DHT, further, via the 3α-hydroxysteroid dehydrogenase (3α-HSD) enzyme, produces neurosteroids – steroids that act in the brain and CNS via rapid, non-genomic mechanisms, including 3α-diol. In the brain, knocking out the AR (null expression) in experimental models causes male rodents to behave in a manner devoid of male sexual and aggressive behavior. [8]. An exhaustive analysis of neurosteroids is beyond the scope of this article and so will not be undertaken.
Aromatase
Aromatization
The enzyme aromatase converts T to E2, and in turn, the activation of the brain’s excitatory neurochemical systems that govern sexual reward motivation (i.e., libido) integrate T and E2. E2 facilitates dopamine (DA) release; and T potentiates the synthesis of nitric oxide (NO) that controls DA release in rats (18). [2]. Thus, endogenous steroid hormones appear to set the stage – [a priming effect] – for increased DA synthesis and release during periods in which sexual responding might be enhanced. (18). [2]. In men, E2 regulates male fertility via the estrogen receptor α (ER-α; ESR1) especially sperm quality a la ERα expression in the testis, efferent ducts, and vas deferens. [7]. To learn about how androgens and their aromatic products (e.g., T and E2) regulate sexual desire, review this author’s article titled Anabolic-Androgenic Steroid Effects on Libido (Part 1: Men).
5α-reductase
5α-amplification
The 5α-reductase enzyme family contains NADPH-dependent oxioreductases that reduce the C-4,5 double bond (Δ⁴) of C-19 and C-21 steroids to 5α-stereoisomers. [5]. Of this family, types 1 and 2 regulate sexual function by neurosteroid (e.g., allopregnanolone) and DHT production a la concentration (or amplification) in CNS and male sex tissues, respectively. [5]. 5α-reductase converts T to DHT in gonadal and CNS tissues (brain and spinal cord) in men, responsible for the growth of male sex organs and male sexual behavior including characteristic male sexual aggression. [4]. From Handelsman, “[t]he amplification pathway is characteristic of the prostate and hair follicle in which testosterone is converted by the type 2 5α-reductase enzyme into the more potent androgen, [DHT]. This pathway produces local tissue-based enhancement of androgen action in specific tissues according to where this pathway is operative… this amplification pathway converts ~4% of circulating testosterone to the more potent, pure androgen, DHT, with its 3 – 10 time greater molar potency in transactivation of the androgen receptor [AR] relative to testosterone.” [1]. Low levels of DHT in male sex tissues results in:
- atrophy of male reproductive tissues
- diminished spermatogenesis and sperm maturation
- diminished sexual function. [9].
3α-HSD
3 alpha-hydroxysteroid dehydrogenase
The complementary activities of 5α-reductase and the enzyme 3α-hydroxysteroid dehydrogenase (3α-HSD), present in the CNS, are crucial for the synthesis of 5α-/3α- reduced neurosteroids, such as 3α-androstanediol, allopregnanolone, and tetrahydrodeoxycorticosterone (THDOC), which control several important neurophysiological mechanisms through allosteric modulation of gamma-aminobutyric acid (GABA) type A receptors. [4].
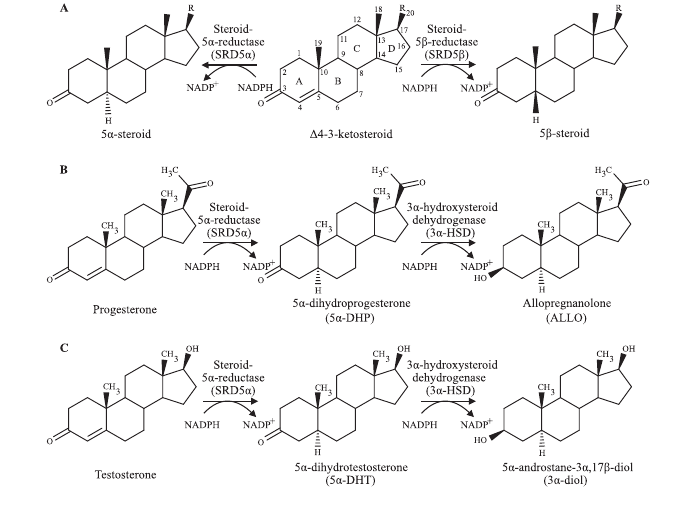
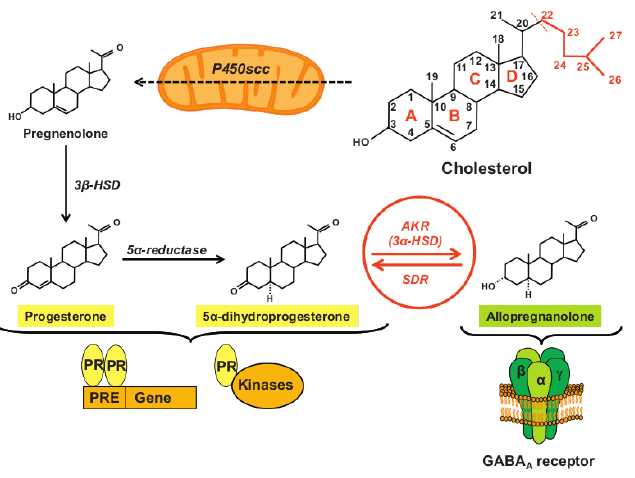
Male reproduction depends on 3α-diol that controls male dominance and sexual behavior. In knockout models of 5α-reductase type 1 (null expression in rodents), T fails to increase aggression and male animals fail to defend territory against other males, dominate females, or engage in intercourse. [5]. Allopregnanolone is a potently neuroactive steroid involved in regulating anxiety, mood, and sexual function. Figure 3. [10].
The Post-Finasteride Syndrome (PFS) is a rare (4% estimated prevalence) but clinically significant persistent condition associated with depression and sexual dysfunction including diminished libido and erectile dysfunction in men who have taken the 5α-reductase inhibitor dutasteride. [11]. These side effects are associated with reductions to pregnenolone, progesterone and its metabolite (i.e., dihydroprogesterone), DHT and E2, and increased levels of DHEA, T, and 3α-diol in the CNS of PFS patients. [12].
Whereas testosterone treatment is associated with increased blood serum and brain levels of allopregnanolone and DHEA, synthetic AAS reduce them, along with their corresponding unconjugated forms and precursors (e.g., 17-hydroxypregnenolone, 5-androstene-3β,17β-diol, progesterone). [13]. [14]. [15].
It is this author’s contention, then, that synthetic AAS, without diversification via aromatase and amplification via 5α-reductase, cannot reliably support sexual function in man because they abrogate the synthesis of vital neurosteroids that regulate it.
Glucocorticoid Modulation
5α-reductase
The type 1 isozyme of 5α-reductase is extensively expressed in the liver where it modulates glucocorticoid clearance. [5]. A lack of 5α-reductase type 1 activity coupled with abrogated T secretion as in the case of administering synthetic AAS sans testosterone concentrates glucocorticoids (e.g., cortisol) in the liver, reducing excretion of glucocorticoids even without significant elevations in blood. [5]. Further, there is evidence in rat that central (i.e., brain) 5α-reduction of T is a requisite step for T’s modulation of glucocorticoids via reduced adrenocorticotropic hormone (ACTH) secretion. [16].
Insulin Sensitivity and Muscle Anabolism
5α-reductase
This accumulation of liver glucocorticoids, in turn, increases gluconeogenesis thereby increasing blood glucose and steatosis, implicating Insulin Sensitivity, by reducing it, causing insulin resistance. [5]. By corollary, then, an important 5α-reductase function is maintained or enhanced insulin sensitivity.
Aromatase
Aromatase is integrally involved in insulin sensitivity regulation, but this is not a unique testosterone effect. To learn about how aromatic products (e.g., E2) regulate insulin sensitivity predominately via action at the ER-α, review this author’s article titled Estrogens and Adipose Tissue and Metabolism section from Estrogen Functions in Male Bodybuilders.
WISP-2
Testosterone increases WISP-2 expression in skeletal muscle. [17]. WISP-2 is a gene that regulates the activities of growth factors (e.g., IGF-I, Notch1, TGF-β) and enzymes involved in extracellular matrix remodeling and metabolism. [18]. [19]. WISP-2 is expressed in human skeletal muscle and fat cells where it is associated with increased lean mass and decreased adipose tissue, regulating insulin sensitivity, fat mass, and extracellular matrix remodeling (i.e., soft tissue metabolism). [19]. To learn about how testosterone regulates connective tissues (e.g., bone, tendons, ligaments), review this author’s article titled Anabolic Steroids and Growth Hormone: Their Impact on Bones, Tendons, Ligaments, and Joints.
Conclusion
In summary, testosterone’s effects are uniquely bioidentical, meaning that it is the only AAS that inherently serves 5α-amplification and aromatization functions. These functions have downstream effects on the brain in regions that are vital for sexual function. Testosterone’s modulation of glucocorticoids depends on brain and liver 5α-amplification where it decreases ACTH secretion and enhances liver clearance of glucocorticoids, respectively. This glucocorticoid modulation overlaps in part with testosterone’s effects on insulin sensitivity and muscle anabolism, where antiglucocorticoid effects enhance insulin sensitivity, but also, testosterone enhances insulin sensitivity, lean body mass, and soft tissue metabolism via increased WISP-2.
References
[1] Handelsman DJ. Androgen Physiology, Pharmacology, Use and Misuse. In: Feingold KR, Anawalt B, Blackman MR, et al., eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; October 5, 2020.
[2] Pfaus, J. G. (2009). Pathways of Sexual Desire. The Journal of Sexual Medicine, 6(6), 1506–1533. doi:10.1111/j.1743-6109.2009.01309.x
[3] Schulster M, Bernie AM, Ramasamy R. The role of estradiol in male reproductive function. Asian J Androl. 2016 May-Jun;18(3):435-40. doi: 10.4103/1008-682X.173932
[4] Patte-Mensah C, Penning TM, Mensah-Nyagan AG. Anatomical and cellular localization of neuroactive 5 alpha/3 alpha-reduced steroid-synthesizing enzymes in the spinal cord. J Comp Neurol. 2004 Sep 20;477(3):286-99. doi: 10.1002/cne.20251
[5] Robitaille J, Langlois VS. Consequences of steroid-5α-reductase deficiency and inhibition in vertebrates. Gen Comp Endocrinol. 2020;290:113400. doi:10.1016/j.ygcen.2020.113400
[6] Simpson ER, Mahendroo MS, Means GD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocrine Rev 1994;15:342-355.
[7] Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in Male Physiology. Physiol Rev. 2017 Jul 1;97(3):995-1043. doi:10.1152/physrev.00018.2016.
[8] Sato, T., Matsumoto, T., Kawano, H., Watanabe, T., Uematsu, Y., Sekine, K., … Kato, S. (2004). Brain masculinization requires androgen receptor function. Proceedings of the National Academy of Sciences, 101(6), 1673–1678. doi:10.1073/pnas.0305303101
[9] Robitaille J, Langlois VS. Consequences of steroid-5α-reductase deficiency and inhibition in vertebrates. Gen Comp Endocrinol. 2020;290:113400. doi:10.1016/j.ygcen.2020.113400
[10] Schumacher, M., Mattern, C., Ghoumari, A., Oudinet, J.P., Liere, P., Labombarda, F., … Guennoun, R. (2014). Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Progress in Neurobiology, 113, 6–39. doi:10.1016/j.pneurobio.2013.09.004
[11] Irwig, M. S. (2014). Persistent Sexual and Nonsexual Adverse Effects of Finasteride in Younger Men. Sexual Medicine Reviews, 2(1), 24–35. doi:10.1002/smrj.19
[12] Melcangi RC, Santi D, Spezzano R, et al. Neuroactive steroid levels and psychiatric and andrological features in post-finasteride patients. J Steroid Biochem Mol Biol. 2017;171:229-235. doi:10.1016/j.jsbmb.2017.04.003
[13] Pluchino N, Ninni F, Casarosa E, Lenzi E, Begliuomini S, Cela V, Luisi S, Freschi L, Merlini S, Giannini A, Cubeddu A, Genazzani AR. Sexually dimorphic effects of testosterone administration on brain allopregnanolone in gonadectomized rats. J Sex Med. 2008 Dec;5(12):2780-92. doi: 10.1111/j.1743-6109.2008.00999.x
[14] Nasimeh Yazdani, Stacy Matthews Branch. Daily subcutaneous testosterone for management of testosterone deficiency. Front. Biosci. (Elite Ed) 2018, 10(2), 334–343. https://doi.org/10.2741/E825
[15] Ruokonen, A., Alén, M., Bolton, N., & Vihko, R. (1985). Response of serum testosterone and its precursor steroids, SHBG and CBG to anabolic steroid and testosterone self-administration in man. Journal of Steroid Biochemistry, 23(1), 33–38. doi:10.1016/0022-4731(85)90257-2
[16] Handa, R. J., Kudwa, A. E., Donner, N. C., McGivern, R. F., & Brown, R. (2013). Central 5-alpha reduction of testosterone is required for testosterone’s inhibition of the hypothalamo-pituitary–adrenal axis response to restraint stress in adult male rats. Brain Research, 1529, 74–82. doi:10.1016/j.brainres.2013.07.021
[17] Ye F, McCoy SC, Ross HH, et al. Transcriptional regulation of myotrophic actions by testosterone and trenbolone on androgen-responsive muscle. Steroids. 2014;87:59-66. doi:10.1016/j.steroids.2014.05.024
[18] Leask, A., & Abraham, D. J. (2006). All in the CCN family: essential matricellular signaling modulators emerge from the bunker. Journal of Cell Science, 119(23), 4803–4810. doi:10.1242/jcs.03270
[19] Alami T, Liu JL. Metabolic Effects of CCN5/WISP2 Gene Deficiency and Transgenic Overexpression in Mice. Int J Mol Sci. 2021 Dec 14;22(24):13418. doi: 10.3390/ijms222413418
About the author
Type-IIx is a physique coach, author, and researcher. Bolus: A Practical Guide and Reference for recombinant Human Growth Hormone Use will be his first published textbook, anticipated for release in early 2023. Ampouletude.com will be Type-IIx's base of operations for coaching services and publications. Type-IIx is proud to be a contributing writer to MesoRx, his home forum, where he is a regular poster.
Leave a Reply
You must be logged in to post a comment.