
The Testosterone Trials were a set of seven randomized placebo-controlled trials that evaluated the efficacy of testosterone replacement therapy (TRT) in older men with low testosterone levels [1]. Trials like these are the pinnacle of clinical science. It’s been commonplace to rely on data from small, low-quality trials, or even just ‘expert opinion’ with regard to TRT. This is unreliable for a great variety of reasons. Moreover, while it can give good clues about certain clinical effects, and the direction of it (e.g. improved libido), it usually is quite bad at estimating the effect size. That is: how well something actually works. And, as often is the case, multiple small, low-quality trials, usually demonstrate inconsistent results.
In 2003, a panel of the American Institute of Medicine (IOM) and National Academy of Sciences recommended conducting good-quality trials to evaluate the efficacy of TRT in the elderly. Because, at the time, the data was largely crap. While it took a couple of years, a large group of researchers, including some big names like Shalender Bhasin, Alvin Matsumoto, and Glenn Cunningham, undertook the tremendous effort to set up these trials. Recruitment started in November 2009, and data collection was finally completed in July 2014.
So what were these seven trials? They looked into clinical endpoints as follows:
-
Physical function trial – The aim was to see if TRT would improve distance on the 6-minute walk test by at least 50 meter. This might sound silly, but walking isn’t self-evident for the elderly. Moreover, it correlates well with various meaningful clinical outcomes.
-
Sexual function trial – Probably doesn’t require much of an introduction. It was to see if TRT improved libido and sexual activity.
-
Vitality trial – Formally speaking, it evaluated whether or not TRT improved scores on the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale (and some other subscales). This scale gives an indication of how well someone with a chronic illness is managing in day to day life, in terms of fatigue, with some questionnaires.
-
Cognitive function trial – What’s in the name: participants were bombarded with a bunch of cognitive tests to see how TRT affected it.
-
Anemia trial – TRT increases red blood cell production (erythropoiesis). Quite in fact, increased hematocrit (the % of blood volume occupied by red blood cells) is the most frequent adverse effect associated with TRT [2]. This is very useful for people who suffer from anemia, as they have too few red blood cells. Thus this trial evaluated whether TRT could improve unexplained mild to moderate anemia (as measured by hemoglobin status).
-
Bone density trial – Osteoporosis is a serious problem in aging. The bones become weak and brittle with a resulting increase in bone fracture risk. This trial evaluated bone mineral density at various sites to see if TRT improved this.
-
Cardiovascular trial – One of the major concerns with TRT is that it might increase cardiovascular disease risk. You’d need a huge trial to adequately address this question. You’d need way more participants than this trial had. So, instead, their primary outcome was the progression of noncalcified coronary artery plaque volume. This can already provide some important clues.
A total of 788 men were recruited for these trials. Each man was eligible to participate in any of these trials in parallel, as long as he met the criteria for it. TRT (testosterone gel) was given for 1 year. The dose was titrated up if testosterone levels were below 500 ng/dL (17.4 nmol/L) and titrated down if testosterone levels were above 800 ng/dL (27.8 nmol/L). Median baseline levels were 234 ng/dL (8.1 nmol/L) and increased to a median of about 500 ng/dL (17 nmol/L; apparently dose titration didn’t go that well). Let’s go over each of these trials to see what they demonstrated. (And let’s see if they fit your beliefs about what TRT does and doesn’t do.)
The physical function trial
As is known, testosterone increases muscle strength and hypertrophy. This is especially evident when supraphysiological dosages are being used. But what about TRT dosages? And how does this translate to something that’s of practical use? That was the concern of this trial [3].
They tried to answer this question by seeing how much the distance of the subjects improved on something called the Six Minute Walk test (6MW) [4]. This test is fairly simple: let someone walk as far as he can in six minutes. The covered distance can be easily measured, you don’t need any special equipment to perform the test, it only takes 6 minutes, and as long as somebody can walk, this person can perform the test. And, of course, the ability to walk (long distances), or plain mobility, is really important for older folks (mind you, the mean age of these men was 72 years). Research had also shown a clear cut-off of how much the 6MW would need to be improved for men (in this case, COPD patients [4]) to consider their performance to have improved: about 50 meters. So around that point you can also consider an improvement to be clinically meaningful. While all men (788 in total) were subjected to this test, they looked at men who had symptomatic and objective mobility disability in particular. A total of 387 subjects qualified for that.
What did the results demonstrate? When looking at the subjects with movement disability, there were 42 % more subjects in the testosterone group than the placebo group who improved their 6MW by at least 50 meters. However, this difference was not statistically significant. When looking at all subjects, there were 77 % more subjects in the testosterone group than the placebo group who improved their 6MW by at least 50 meters. This was a statistically significant difference.
Woah, impressive. Right? Actually, these percentages sound more impressive than they are: the vast majority of men didn’t clinically significantly improve their 6MW distance. For those who completed the trial, 35 out of 172 with movement disability improved with testosterone, and 20 out of 165 improved with a placebo. When looking at all participants, 71 out of 346 improved with testosterone, whereas 41 out of 326 improved with placebo. There thus only seems to be a small minority of older hypogonadal men who benefit clinically significantly from TRT in terms of physical function.
The sexual function trial
Decreased sexual function is a common complaint in hypogonadism, especially in severe hypogonadism. At the time when this trial was conducted, it was clear that TRT improved sexual function in severe hypogonadism and in young to middle-aged adults. The evidence for older men, however, was less compelling.
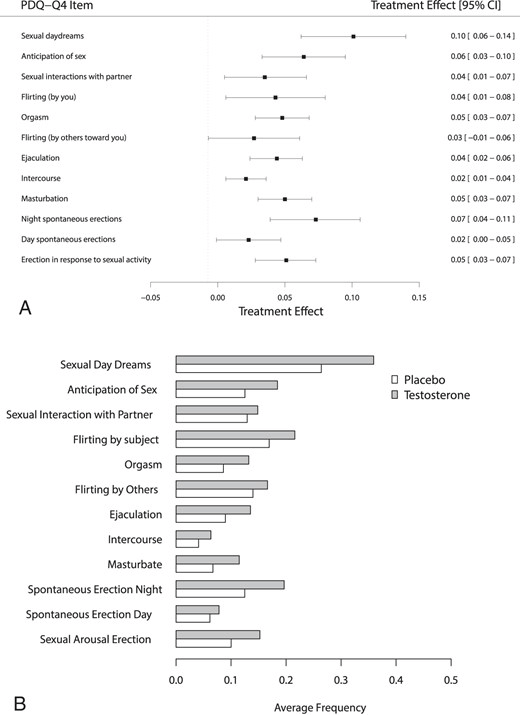
The sexual function trial assessed sexual activity, sexual desire, and erectile function and by means of questionnaires and interviews [5]. Overall, there was a moderate effect on sexual activity, with statistically significant improvements seen in 10 of the 12 measures of it:
There were also statistically significant improvements in sexual desire and erectile function. These improvements were small to moderate.
Not much new here, although some proclaim that TRT has a huge impact on sexual function and erectile function.
Vitality trial
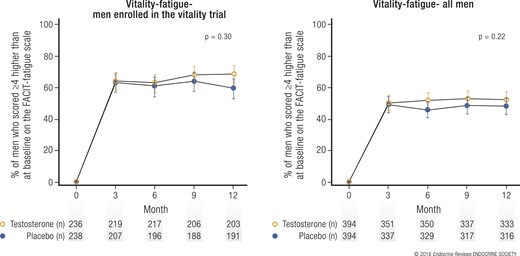
When looking at the FACIT-fatigue scale, there were about as many men on testosterone who improved with at least 4 points on this scale as there were men who were given a placebo. The fun thing here is that in both groups, the majority of men improved. The power of placebo! Here captured in an image:
Either way, when looking specifically at improvements on vitality (determined using the SF-36 vitality subscale), mood, and depressive symptoms, a small, but statistically significant increase, was observed.
The crux here is that testosterone per se might increase vitality by only a small margin, yet the power of suggestion alone might lead to the biggest improvement.
Cognitive function trial
Cognitive decline is a serious problem affecting the elderly. The researchers hypothesized, based on some hints from the literature, that TRT might improve cognitive function or slow down its decline. Therefore, in this trial [6], they looked at the effects of testosterone on cognitive function, such as verbal memory (memorizing spoken or written information), visual memory (being able to recall visual inputs, such as shapes, letters, or practically anything visual), executive function, and spatial ability.
So what did TRT do? I can keep this one short: nothing.
Anemia trial
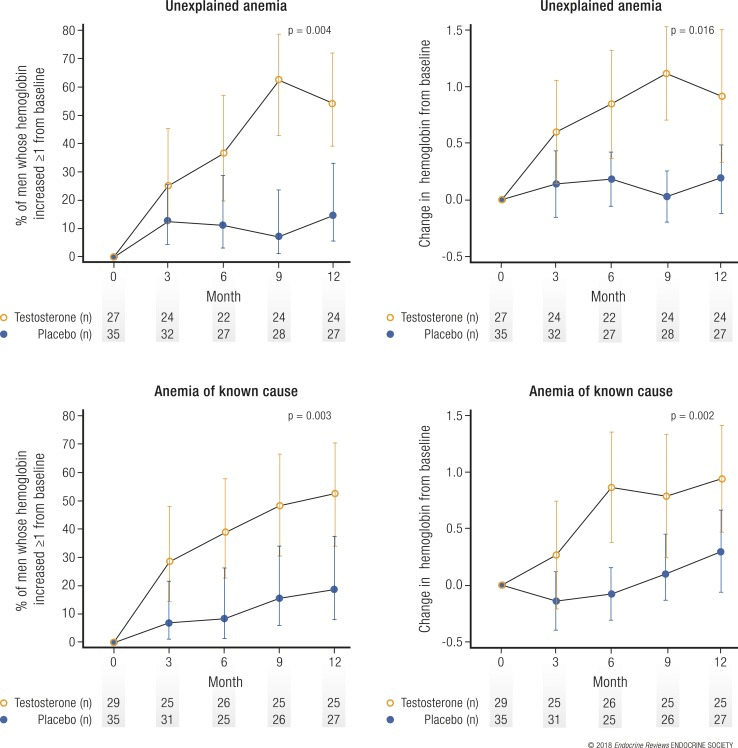
Testosterone, or I should say androgens in general, are quite effective in increasing red blood cell production, a process known as erythropoiesis. I’ve extensively covered the increase in hemoglobin (a key protein in red blood cells) as a result of anabolic steroid (ab)use in a previous article. In general, the increase in red blood cells is unwanted. However, the situation is, of course, different for people who suffer from anemia: a low level of red blood cells. For these patients, TRT might be super awesome, as it has the potential to normalize red blood cells.
Did the anemia trial [7], indeed, demonstrate this? Yes, it did. The increase was modest, with a mean increase of 0.8 to 1.1 g/dL (or roughly 3 % point in hematocrit) at the 6- to 12-month mark. There is reason to assume this is a clinically relevant increase. Roughly half the men with anemia, both of explained and unexplained cause, saw an increase in their hemoglobin levels of at least 1 g/dL in the testosterone-treated group, whereas a little over 10 % of those in the placebo group witnessed such an increase.
Bone density trial
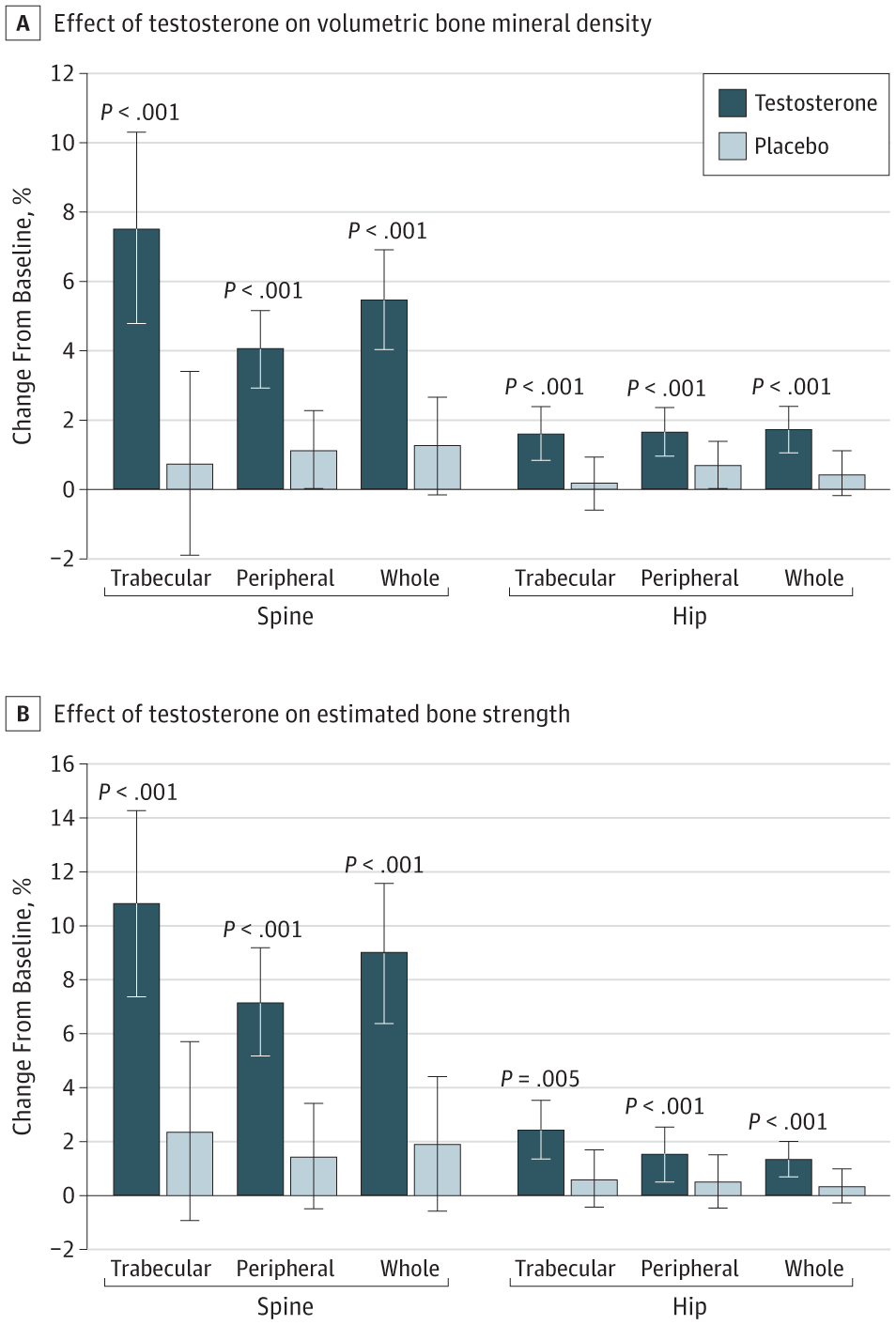
Another issue with aging men (as well as women) is the decrease in bone mineral density that takes place. Consequently, this increases the risk of fractures. It’s well-established that a (severe) testosterone deficiency accelerates the decrease in bone mineral density that takes place with aging. This is especially evident, for example, with men receiving GnRH agonists as a means of androgen-deprivation therapy for prostate cancer treatment [8].
In the bone density trial, testosterone intervention increased volumetric trabecular bone mineral density of the lumbar spine and estimated bone strength compared to a placebo.
However, while this is all good news, it’s not entirely sure yet that this would also decrease fracture risk. A longer, more extensive trial would be required for this. Nevertheless, the outlook is promising. (And it certainly would be my expectation.)
Cardiovascular trial
Perhaps the trial that has received the most attention of them all, and also is the one most looked into by the anabolic steroid community. The researchers hypothesized that TRT would slow the progression of noncalcified coronary artery plaque volume [10]. They measured the coronary artery plaque volume with coronary CT angiography. Plaque-formation in the coronary arteries is a big problem. It narrows the lumen of the coronary arteries, thereby impeding blood flow to the heart. If the plaque surface ruptures, a blood clot might form which can possibly completely block off blood flow in the artery. Hello heart attack.
So what did this trial show? Unfortunately, it showed a significant increase in coronary artery plaque volume in the TRT group compared to the placebo group. Noncalcified coronary artery plaque volume increased from 204 to 232 mm3 in the TRT group, and from 317 to 325 mm3 in the placebo group. Total plaque volume increased significantly more in the TRT group too.
The study received some (unfounded) critique that the differences at baseline explain the results, but this most likely simply is not true. There are multiple reasons for this that the researchers have outlined themselves too:
-
All analyses were adjusted for baseline values as continuous variables
-
There was absolutely no association between baseline plaque volume and the change from baseline in a linear regression model
-
A scatterplot of the change in volume showed no association
-
The effect was similar in men whose baseline plaque volume was less than the median compared to those whose baseline plaque volume was greater than the median
Either way, these results are concerning. However, it’s impossible to tell how this translates to a potential increased risk of cardiovascular disease. A really large trial, which lasts several years, needs to be set up to accurately assess the change in cardiovascular disease risk as a result of TRT. Most lines of evidence, including randomized-controlled trials, suggest there’s no change in cardiovascular disease risk [11]. What should be kept in mind is that all these randomized-controlled trials so far have been statistically underpowered, and too short in duration, to actually measure this. As such, there’s currently a large trial underway which is examining this. This trial, called the TRAVERSE trial, will involve 6,000 hypogonadal subjects between the ages of 45 and 80 years old that will be randomized to TRT or a placebo, for a duration of 5 years. Recruitment of the first subjects started back in 2018, and they’re currently still recruiting subjects (you can imagine this can take quite some time to get enough subjects). The researchers are looking at major adverse cardiac events, as well as prostate safety. The expected completion date is somewhere in 2022. So the final subjects will likely complete the trial somewhere in 2027. So it will probably take at least till 2028 before the results will be published in the literature (perhaps intermittent results will be released earlier). Till then, the effect of TRT on cardiovascular disease risk remains an outstanding question.
References
-
Snyder, Peter J., et al. “The Testosterone Trials: seven coordinated trials of testosterone treatment in elderly men.” Clinical trials 11.3 (2014): 362-375.
-
Calof, Olga M., et al. “Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials.” The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 60.11 (2005): 1451-1457.
-
Snyder, Peter J., et al. “Effects of testosterone treatment in older men.” New England Journal of Medicine 374.7 (2016): 611-624.
-
Redelmeier, Donald A., et al. “Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients.” American journal of respiratory and critical care medicine 155.4 (1997): 1278-1282.
-
Cunningham, Glenn R., et al. “Testosterone treatment and sexual function in older men with low testosterone levels.” The Journal of Clinical Endocrinology & Metabolism 101.8 (2016): 3096-3104.
-
Resnick, Susan M., et al. “Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment.” Jama 317.7 (2017): 717-727.
-
Roy, Cindy N., et al. “Association of testosterone levels with anemia in older men: a controlled clinical trial.” JAMA internal medicine 177.4 (2017): 480-490.
-
Wadhwa, Vivek K., et al. “Long‐term changes in bone mineral density and predicted fracture risk in patients receiving androgen‐deprivation therapy for prostate cancer, with stratification of treatment based on presenting values.” BJU international 104.6 (2009): 800-805.
-
Snyder, Peter J., et al. “Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial.” JAMA internal medicine 177.4 (2017): 471-479.
-
Budoff, Matthew J., et al. “Testosterone treatment and coronary artery plaque volume in older men with low testosterone.” Jama 317.7 (2017): 708-716.
-
Gagliano-Jucá, Thiago, and Shehzad Basaria. “Testosterone replacement therapy and cardiovascular risk.” Nature Reviews Cardiology 16.9 (2019): 555-574.
About the author
Peter Bond is a scientific author with publications on anabolic steroids, the regulation of an important molecular pathway of muscle growth (mTORC1), and the dietary supplement phosphatidic acid. He is the author of several books in Dutch and English, including Book on Steroids and Bond's Dietary Supplements.

Leave a Reply
You must be logged in to post a comment.