
Anabolic to androgenic ratio: an introduction
Anabolic-androgenic steroids (AAS) have anabolic and androgenic properties, hence the name. In general, the anabolic properties are considered to be the muscle-building effect and stimulatory effect on bone mineral density (BMD). Most of their other effects are considered to be the androgenic effects, such as their effects on the prostate, bone marrow, heart, hypothalamus and pituitary, etc. In general, these effects are considered to be unwanted. For example, a meta-analysis found an increase in hematocrit (the % of blood volume occupied by red blood cells) to be the most frequent adverse effect associated with testosterone replacement therapy [1]. Another issue of androgens is that they’re able to induce growth of prostate cancer. As such, one of the therapies used in prostate cancer is androgen deprivation therapy. However, it’s important to note that there’s a limit to the ability of androgens to stimulate prostate cancer growth. Meaning that, up to a certain concentrations, androgens will stimulate growth, but above it, they will have little or no further effect. The androgen receptors get saturated. And this is actually something that seems to already take place at low testosterone concentrations, in the low hypogonadal range (this is described by the Saturation Model [2]) . I’ll get back to this point later again when I’ll be discussing a renowned assay that is used for assessing the anabolic to androgenic ratio.
The idea of the anabolic to androgenic ratio is to provide a ratio of how AAS compare relative to each other in terms of their anabolic and androgenic potency. So for example, one could take testosterone as the “index” steroid, assigning it an anabolic to androgenic ratio of 100 to 100 (or just 1). Then say through some experiment it’s determined that another steroid has a ratio of 400:200 (or just 2) This would imply that each molecule of this steroid is 4 times as anabolic as testosterone, while only being twice as androgenic.
Pretty appealing, right? If you want to minimize the risk of androgenic side effects, you can just select an AAS with a very favorable anabolic to androgenic ratio, and you’re all set. However, there are so many issues with both the concept of an anabolic to androgenic ratio, as well as how it’s experimentally determined, that all these ratios you’ll find online or in the literature are pretty damn worthless.
The Hershberger assay
A very common assay that’s used to determine the anabolic to androgenic is the so-called Hershberger assay. The assay was first described in 1953 by Hershberger and his colleagues at the University of Wisconsin [3]. The assay works as follows. You get a bunch of rats and you castrate them. The castration ensures that they have very little endogenous testosterone which could affect results. Following this, you administer the AAS you want to know the anabolic to androgenic ratio of. Then you wait a little while (8 days in the case of the original Hershberger paper) and then you kill these rats in order to dissect them and weigh the levator ani muscle, the ventral prostate, and the seminal vesicles. The weight increase of the levator ani would then be indicative of the AAS’ anabolic activity, whereas that of the ventral prostate and seminal vesicles would be indicative of its androgenic activity.
While this sounds reasonable, there are a number of problems with this assay. An initial point I would like to touch on involves the levator ani muscle itself, which, in fact, is the dorsal bulbocaverous muscle [4]. It’s a muscle that’s part of the male reproductive system and therefore shouldn’t be considered representative of skeletal muscle by any means. It’s a strongly androgen-dependent muscle and after castration it undergoes a rate of weight decrease that’s similar to that of denervation atrophy in skeletal muscles [5]. Just this information alone already ensures the anabolic side of the ‘equation’ is flawed. Another problem is that the dorsal bulbocavernous muscle and seminal vesicles respond differently to a decrease in the AAS concentration within the phsyiological range [6]. As a result, the experimentally determined ratio will hinge on the dose used as well as the point in time the measurements are taken. This is illustrated well in the figure below taken from a publication by van der Vies [6]. During the first 3 days, the concentration of the AAS (nandrolone in this case) is high enough to stimulate growth of both the seminal vesicles as well as the dorsal bulbocavernous muscle. However, after three days the concentration is not high enough to sustain this growth for the seminal vesicles, and they decrease in size again. Nevertheless, the bulbocavernous muscle is still stimulated sufficiently to continue growing in size. Thus, if you’d determine the anabolic to androgenic ratio on day 3 it would be a whole lot different from when you’d determine it on day 7, despite it being the same compound.
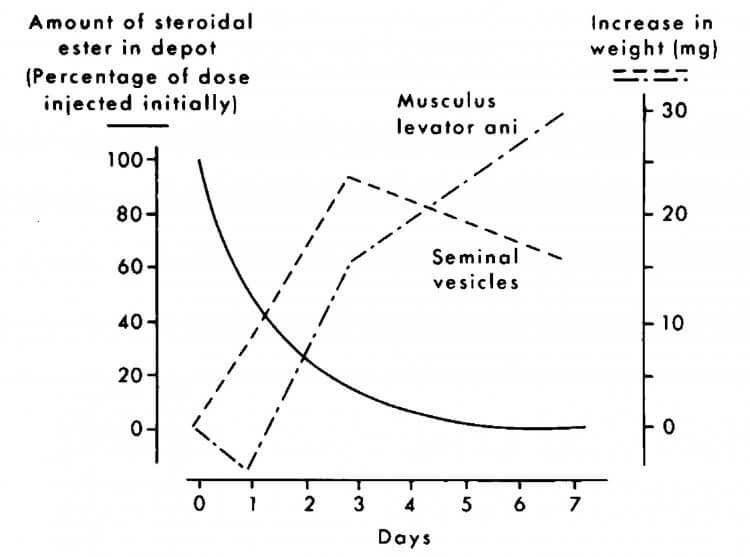
This also highlights that different organs simply respond differently depending on the concentration of the compound. And even if there would be an accurate way of determining an anabolic to androgenic ratio, extrapolating it beyond physiological concentrations would be completely flawed.
Another flaw is the assumption that growth of the ventral prostate or seminal vesicles is representative of all other androgenic effects. There’s never been any evidence to support this assumption. Androgenic tissues vary quite a lot from each other and it’s definitely not to be expected that one tissue responds to the same extent as another. Quite in fact, remember what I mentioned in the introduction about the prostate? Androgens already appear to stop further stimulating the prostate beyond the low hypogonadal range. Indeed, prostate volume remains unchanged when healthy men are given 600 mg of testosterone enanthate weekly for 20 weeks [7]. Yet we know for a fact that other androgenic side effects start to pop up when dosing that high! Anyhow, this also highlights the issue of the concept of the anabolic to androgenic ratio itself. A single ratio is never able to capture the differential responses of various tissues to androgens or the complexity of the androgenic response within a specific tissue, to be of value. Different tissues respond differently to AAS, how is that ever gonna be represented by a single number?
Of course, the Hershberger assay is carried out in rats, not humans (thank god though). It’s yet another flaw to assume that the homologues tissues in humans would respond in the same way as in the rat to AAS. The whole Hershberger assay is just stacked with flaws and it’s beyond me while this assay is still being used today to screen for potential selective androgen receptor modulators (SARMs). For example, GlaxoSmithKline assessed the tissue selectivity of their SARM GSK2881078 using the classic Hershberger assay [8].
Relative binding affinity (RBA) assay
While the Hershberger assay is performed in a living organism, the relative binding affinity (RBA) assays are performed in a petri dish sortasay. It looks at the binding affinity of compounds for the androgen receptor (AR). In this context, binding affinity refers to how strongly an anabolic steroid binds to the AR. The RBA thus means how strongly one anabolic steroid binds to the AR compared to another. Or in other words: relative to each other.
The principle is quite simple. You get a reference steroid, commonly methyltrienolone (R1811), and measure its binding affinity. Subsequently you measure the binding affinity of other steroids, and you express the data relative to this reference steroid. So methyltrienolone is given an RBA of 1, being the reference compound, and then if something else binds twice as strong its assigned an RBA of 2. Similarly, if something else binds twice as weak its assigned an RBA of 0.5. You could do these measurements in different cell types. One representing skeletal muscle and another representing somehow its androgenic effects (e.g. prostate cells). As such, this comes with some of the same objections as the Hershberger assay as described above.
Anyway, I’ve compiled the RBAs of a selection of popular AAS as measured in rat and rabbit tissues in the table below [9]. If you were to go off by these results, testosterone would have a more favorable anabolic to androgenic ratio than nandrolone. Funny huh? That’s kind of the opposite of what they’re seeing in the Hershberger assays. It’s also a bit surprising, given that testosterone’s androgenic effects are amplified in tissues expressing 5α-reductase, because of the conversion to the more potent androgen DHT. Conversely, nandrolone’s androgenic action is weakened in tissues expressing this enzyme, because of the conversion to the less potent androgen dihydronandrolone (DHN) [10].
| Competitor | Rabbit muscle | Rat muscle | Rat prostate | Ratio rat muscle to prostate |
| Methyltrienolone | 1 | 1 | 1 | 1 |
| DHT | 0.07 | <0.01 | 0.46 | 0.03 |
| 1α-methyl-DHT | 0.21 | 0.08 | 0.25 | 0.32 |
| Testosterone | 0.07 | 0.23 | 0.15 | 1.53 |
| Nandrolone | 0.20 | 0.24 | 0.60 | 0.40 |
Another thing this data aptly reveals are the interspecies differences of RBA values. In rat muscle, 1α-methyl DHT binds about 3x as weak to the AR as does testosterone. If you look at the data in rabbit muscle, you basically see the opposite: 1α-methyl-DHT binds roughly 3x as strong to the AR as does testosterone. Extrapolating from one animal species to another is (highly) problematic, and thus also extrapolating from the rat, rabbit, or whatever animal, to humans.
In case you’re wondering why DHT demonstrates such a low RBA in rabbit and rat muscle, this is most likely due to its rapid breakdown in msucle tissue. DHT forms an excellent substrate for the enzyme 3α-HSD. This enzyme breaks it down to 3α-androstanediol, which binds very weakly to the AR [11]. This also happens in humans [12], and I guess this is why you don’t see anyone cycle with DHT. It’s pretty darn ineffective, as it gets broken down in muscle tissue.
A final point that needs to be made is that binding affinity doesn’t dictate its potency to also modulate gene expression. Which is ultimately what it’s all about. However, this is something you can experimentally assess too. This is something that androgen responsive reporter gene assays (AR bioassays) do. These bioassays have, initially as far as I know, been used to screen for novel designer androgens in urine samples to counteract doping. An AR bioessay essentially is able to demonstrate if a sample contains something that manages to activate the androgen receptor and initiate gene transcription. In order to counteract doping, this is very useful. After all, you can show that a urine sample contains something that activates the AR without knowing the chemical structure of the compound used.
Anyways, one such assay was developed by a team of Dutch scientists [13]. The scientists made use of an assay called the Androgen Responsive Chemical Activated LUciferase eXpression (AR CALUX) bioassay. They took a human osteosarcoma cell line and co-transfected it with the human AR and a luciferase reporter gene that is under transcriptional control of androgen response elements (AREs). This means that when the AR gets activated, the enzyme luciferase comes to expression. This enzyme produces bioluminescence, or to phrase it simply: light. And light can be measured. Thus the degree of bioluminescence is the degree to which androgen receptor activation is taking place.
The scientists then proceeded to test a variety of known AAS with the AR CALUX bioassay. Similar to the RBA, you can calculate the relative potency in terms of receptor activation (REP). And not only have the scientists done this for the AR, they’ve also done this for the progesterone receptor (PR), both isoforms of the estrogen receptor (ERα and ERβ) and the glucocorticoid receptor (GR). I’ve listed the REP of some (popular) AAS in the table below.
| Compound | AR REP | PR REP | ERα REP | ERβ REP | GR REP |
| DHT | 1.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 |
| Testosterone | 0.2135 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Methyltrienolone | 1.0625 | 0.3281 | 0.0000 | 0.0000 | 0.0000 |
| Trenbolone | 0.9434 | 0.0261 | 0.0000 | 0.0000 | 0.0000 |
| Stanozolol | 0.1915 | 0.0000 | 0.0002 | 0.0006 | 0.0000 |
| Methandienone | 0.0721 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Nandrolone | 0.6987 | 0.0050 | 0.0001 | 0.0002 | 0.0000 |
| Boldenone | 0.1072 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Oxandrolone | 0.0124 | 0.0000 | 0.0001 | 0.0001 | 0.0000 |
Let me highlight testosterone’s and DHT’s REP for the AR. Testosterone’s REP is about 5x lower than DHT’s REP. What does that tell us? That DHT isn’t broken down in the cell line they used as would be the case in the real world in skeletal muscle. The metabolism that usually takes place in skeletal muscle thus doens’t seem to occur in this cell line. This issue invalidates the results of this bioassay for those AAS that are metbaolized in skeletal muscle, such as DHT, but likely also methenolone (Primobolan). Another issue is that gene expression is complex (and that’s an understatement still). The AR regulates a vast number of genes. If two compounds increase gene transcription of a certain gene to a similar degree, it doesn’t necessarily mean that these two compounds comparably modulate gene transcription of other genes. Surely it wouldn’t be surprising if there’s a correlation to one degree or another, but these bioassays just paint a rough picture. Although it’s likely that this rough picture is more accurate than that of RBAs. Nevertheless, to date, AR bioassays have not been performed in multiple cell lines of various (androgen-sensitive) tissues to provide us with ‘new’ anabolic to androgenic ratios.
Conclusion
Both the Hershberger assay as well as studies assessing the RBAs of AAS in various tissues are severly flawed. Moreover, the concept of a number capturing the complexity of anabolic to androgenic properties should be abondend. A single ratio is simply unable to capture the differential responses of various tissues to androgens, as well as the complexity of the androgenic response within a specific tissue, to be of value. Perhaps a ‘profile of activity’ that describes the androgenic action on a per tissue basis would be more appropriate. Something like this has been proposed to describe what an ideal SARM would look like for treating a specific condition. However, it’s exceedingly hard to quantify androgenic action per tissue—if not impossible. Perhaps that AR Bioassays performed in cell lines of tissues of interest might hold some predictive clinical value. In the end, after all, clinical trials are required to demonstrate (the degree of) adverse events that occur with usage of a certain compound.
References
- Calof, Olga M., et al. “Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials.” The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 60.11 (2005): 1451-1457.
- Morgentaler, Abraham, and Abdulmaged M. Traish. “Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth.” European urology 55.2 (2009): 310-321.
- Hershberger, L. G., Elva G. Shipley, and Roland K. Meyer. “Myotrophic activity of 19-nortestosterone and other steroids determined by modified levator ani muscle method.” Proceedings of the Society for Experimental Biology and Medicine 83.1 (1953): 175-180.
- Hayes, Keith J. “The so-called ‘levator ani’ of the rat.” European Journal of Endocrinology 48.3 (1965): 337-347.
- Gori, Zina, C. Pellegrino, and Maria Pollera. “The castration atrophy of the dorsal bulbocavernosus muscle of rat: an electron microscopic study.” Experimental and molecular pathology 6.2 (1967): 172-198.
- Van der Vies, J. “Implications of basic pharmacology in the therapy with esters of nandrolone.” European Journal of Endocrinology 110.3_Suppla (1985): S38-S44.
- Bhasin, Shalender, et al. “Effect of testosterone supplementation with and without a dual 5α-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial.” Jama 307.9 (2012): 931-939.
- Neil, David, et al. “GSK2881078, a SARM, produces dose-dependent increases in lean mass in healthy older men and women.” The Journal of Clinical Endocrinology & Metabolism 103.9 (2018): 3215-3224.
- Saartok, Tönu, Erik Dahlberg, and JAN-ÅKE GUSTAFSSON. “Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin.” Endocrinology 114.6 (1984): 2100-2106.
- Bergink, E. W., et al. “Comparison of the receptor binding properties of nandrolone and testosterone under in vitro and in vivo conditions.” Journal of steroid biochemistry 22.6 (1985): 831-836.
- Jin, Yi, and Trevor M. Penning. “Steroid 5α-reductases and 3α-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism.” Best Practice & Research Clinical Endocrinology & Metabolism 15.1 (2001): 79-94.
- Becker, H., et al. “In vivo uptake and metabolism of 3H-testosterone and 3H-5α-dihydrotestosterone by human benign prostatic hypertrophy.” European Journal of Endocrinology 71.3 (1972): 589-599.
- Houtman, Corine J., et al. “Detection of anabolic androgenic steroid abuse in doping control using mammalian reporter gene bioassays.” Analytica chimica acta 637.1-2 (2009): 247-258.
About the author
Peter Bond is a scientific author with publications on anabolic steroids, the regulation of an important molecular pathway of muscle growth (mTORC1), and the dietary supplement phosphatidic acid. He is the author of several books in Dutch and English, including Book on Steroids and Bond's Dietary Supplements.

Leave a Reply
You must be logged in to post a comment.