In my previous article I discussed the more conventional treatment modalities for androgenetic alopecia, namely the oral and topical versions of finasteride and minoxidil. In this article I’ll go over some of the more novel or experimental ones, such as topical androgen receptor antagonists, platelet-rich plasma (PRP) therapy, Wnt signaling modulators and prostaglandins.
Androgen receptor antagonists
The approach of androgen receptor antagonists is similar to that of 5α-reductase inhibitors such as finasteride and dutasteride: block androgenic action. The mechanism is different however. 5α-reductase inhibitors block the conversion of testosterone to the more potent androgen dihydrotestosterone (DHT). As such, testosterone’s androgenic effect won’t be amplified in scalp tissue. Androgen receptor antagonists block androgenic action by preventing androgens from binding to their receptor. As such, their action is blocked at the level of the androgen receptor itself, and thus it targets virtually all androgens rather than specific ones, as is the case with 5α-reductase inhibitors. The problem with this is that its effects really need to stay localized to the scalp. Blocking overall androgen action in other tissues, such as muscle tissue, is definitely unwanted.
One such drug that’s currently undergoing clinical trials is clascoterone (Breezula). It’s being researched by the pharmaceutical company Cassiopea S.p.A. In vitro research in human dermal papilla cells demonstrated the compound to be effective at inhibiting androgen action [1]. It did so to a greater extent than enzalutamide, another androgen receptor antagonist that’s used in the treatment of prostate cancer, and to a comparable extent as finasteride. It’s affinity for the androgen receptor is relatively low, around a 100-fold lower than DHT’s affinity for the androgen receptor [2]. This isn’t really a problem, you can make up for that by just making sure the hair follicle cells are exposed to a high enough concentration of the compound. However, this does beg the question what its affinity is for other steroid receptors, such as the glucocorticoid receptor. If it doesn’t have a high enough specificity for the androgen receptor, you can get off-target effects by binding to these other receptors. In turn, this can lead to side effects. Again, this isn’t necessarily a problem either if systemic exposure is minimal to non-existent.
In August 2020, the FDA approved clascoterone cream 1 % (Winlevi) for the treatment of acne vulgaris in patients 12 years and older [3]. So it’s in the line of expectations that they’re pursuing approval for androgenetic alopecia too. Indeed, in 2019 they completed a phase 2 study with 404 men for the treatment of androgenetic alopecia (EudraCT #2016-003733-23).
Subjects were treated with a 2.5, 5.0, or 7.5 % 1 mL solution of clascoterone which was to be applied twice daily, or 0.0 (vehicle) and 7.5 % once daily, or vehicle twice daily, for a year. While the results haven’t been published in the scientific literature (yet?), the results can still be viewed online in the EU Clinical Trials Register. Total area hair counts increased significantly compared to the vehicle solution group in all treatment groups. (Mostly because the vehicle solution group saw a significant decrease in total area hair count, reflecting the progression of androgenetic alopecia.) Interestingly, hair growth assessment ratings were similar between all groups, although an increase was reported slightly more often in the treatment groups (56.1 to 61.8 % of subjects compared to 50.0 % in the vehicle-only group). Adverse events were similar across groups.
Systemic exposure for the 1 % cream used for the treatment of acne is minimal [4]. Data for their topical solution isn’t available in the published literature, unfortunately. Sexual side effects weren’t tracked in their trial, so it’s hard to derive any potential system exposure on the basis of those results.
As a final note: it’s interesting to see that a trial has been completed in 2016 in which a clascoterone solution was compared to a minoxidil 5 % solution or a placebo for treatment of androgenetic alopecia (NCT02279823). The results have never been published in the scientific literature. There can be a number of reasons for this, but perhaps the most obvious one—from the perspective of a pharmaceutical company—is: disappointing results. I have a feeling it didn’t do that well compared to minoxidil.
Another androgen receptor antagonist that’s making rounds on the internet is RU58841 (also known as PSK-3841 or HMR-3841). It was in phase II trials in 2004, but drug development has discontinued since. At the time, it was under research by Proskelia, the French unit of ProStrakan Group. Proskelia was later acquired by Galapagos in 2006. Importantly, clinical trial results have never been reported in the literature. It’s rumored that this had a financial reason. This sounds plausible, Proskelia was a relatively small company (since it was acquired for 16.5 million USD in 2006). Phase 3 clinical trial costs are very high. You’re looking at a few thousand USD per subject at least (on average they cost several tens of thousand USD per subject). If you multiply that by the, ball park, 1000-2000 subjects you need for such a trial, it quickly becomes apparent that they most likely had to rely on investors to accomplish this. Nevertheless, if Galapagos was interested in this compound, they could have easily funded a phase 3 trial. It should be kept in mind that the primary reason for drugs not going into phase 3 trials is lack of efficacy or safety.
It displays a high affinity for the human androgen receptor, a little lower than the affinity testosterone has for it (which is remarkable, as most antagonists usually have an affinity substantially lower) [5]. Some data of animal trials has been published. It shows an efficacy similar to that of finasteride in stump-tailed macaques [6]. In female testosterone-conditioned nude mice, xenografted scalp tissue from balding men showed more favorable results when compared to controls [7]. Honestly, these trials are preclinical for a reason: they only provide an indication of whether or not it might be interesting to pursue clinical trials. They don’t really provide information beyond that, so I’m mostly mentioning them for completeness. Without clinical trial data nothing much can be said about this compound.
A final remark I would like to make is that it’s suggested that RU58841 can affect the androgen receptor allosterically [8]. Which means as much as that it affects its function by binding at a different site than the ligand-binding site (where androgens would bind). This has a very important practical implication. If there’s competitive binding, its efficacy depends on the concentration of other ligands (such as DHT). With allosteric binding, this is not the case, thus it’s effect is independent of ligand concentrations—which would be ideal for anabolic steroid users as the supraphysiological doses used then wouldn’t affect its efficacy. Unfortunately, I’m unable to access the original study which posits this feature.
Another topical androgen receptor antagonist is fluridil, also known as topilutamide, and sold under the brand name Eucapil. It’s approved for cosmetic use in the Czech republic. One small-scale clinical trial has been published, but its results don’t look promising [9]. 43 subjects with androgenetic alopecia were randomized to receive either a topical 2 % fluridil or placebo solution for 9 months. Counts of hairs in the anagen or telogen phase were made at 0, 3, 6 and 9 months. While there was a larger increase in hairs in the anagen phase and a larger decrease in hairs in the telogen phase in the fluridil group compared to placebo at the 3-month mark, there was no significant difference at the 9-month mark. (They were essentially the same at the 9-month mark.) That’s pretty disappointing. Future trials (which I don’t expect at this point) might elucidate whether this was a peculiarity of the trial or not. One might speculate that a higher concentration of fluridil solution might be required for it to be effective.
Platelet-rich plasma (PRP) therapy
I think a little introduction about platelet-rich plasma (PRP) is in place. What is it actually? In essence it’s a concentrate of a blood with a high concentration of platelets and the red blood cells removed. It’s produced by a process called differential centrifugation [9]. The produced PRP contains a platelet concentration that’s about 2 to 8 times higher than whole blood. The concentration achieved depends on the device and method that’s used. Usually, roughly 30 mL of blood is drawn to prepare PRP.
Platelets are important for coagulation but also contain a variety of growth factors and cytokines [10]. These signaling molecules are the reason for which they’re employed in various medical fields, including dermatology in for example the treatment of androgenetic alopecia. The platelets release these growth factors and cytokines upon activation, which can either occur after injection into the scalp by the body or can be done by adding calcium salts or thrombin to the platelets before injection. It’s thought that these growth factors act on cells of the hair follicle and thereby exert their beneficial effect in treatment of (androgenetic) alopecia.
Given that there’s no standardized procedure of how PRP should be applied, trials can demonstrate different results as a result of differing PRP procedures. While good evidence is lacking, it’s believed that pre-activating the platelets before injecting, and preparing the PRP by utilizing a so-called double-spin protocol, leads to better results.
A 2020 meta-analysis evaluated the effects of PRP therapy as a treatment for androgenetic alopecia [11]. It included 30 randomized-controlled trials for qualitative analysis and 5 of those could be used for quantitative analysis. PRP therapy was effective in increasing hair density as well as hair thickness. In fact, it appeared more effective than minoxidil and finasteride. Whereas a 2017 meta-analysis found finasteride and topical 5 % minoxidil to increase hair density by 18 and 15 hairs per square cm, respectively, PRP therapy led to a mean increase of 33 hairs per square cm.
Adverse events were reported in half the trials and were limited to pain, local erythema (redness) and edema, pinpoint bleeding, and transient headaches, drowsiness, hematomas and scalp sensitivity. No serious adverse events were reported. All in all, PRP therapy is very promising.
Wnt/β-catenin pathway modulators
The Wnt/β-catenin pathway is involved in numerous cellular processes. And, as it turns out, the pathway is also involved in hair follicle growth and development [12, 13]. The canonical pathway involves binding of a Wnt protein to Frizzled—its cell-surface receptor—and its co-receptor LDL-receptor-related protein (LRP) [14]. This triggers a molecular play of several proteins that are involved in the degradation of a protein called β-catenin that modulates gene transcription.Without Wnt signaling, β-catenin is continuously broken down, whereas with activation by a Wnt protein, β-catenin starts to accumulate in the cytosol. β-catenin then translocates to the nucleus where it will stimulate the transcription of Wnt target genes.
A Wnt activator that’s currently under investigation for the treatment of androgenetic alopecia is SM04554 (also known as Dalosirvat). It’s developed by Biosplice Therapeutics (formerly known as Samumed) and 3 clinical trials have been registered and completed: NCT02275351, NCT02503137, NCT03742518. These NCT numbers can be looked up on www.clinicaltrials.gov in order to view their details. The trial registered under number NCT03742518 is a phase II/III trial with 675 participants who were randomized into three groups. One group used a 0.15 % SM04554 solution once daily, another used a 0.25 % solution once daily, and the third group received a vehicle solution. The trial lasted 48 weeks and was completed on the 31st of December 2020. Unfortunately, no clinical trial results have been published in the scientific literature (yet). However, when doing some digging on Google, you can find some slides that were used during a presentation at the International Dermatology and Cosmetology Congress (INDERCOS) in March 2019. Some results of a phase II trial are presented in it, including this slide:
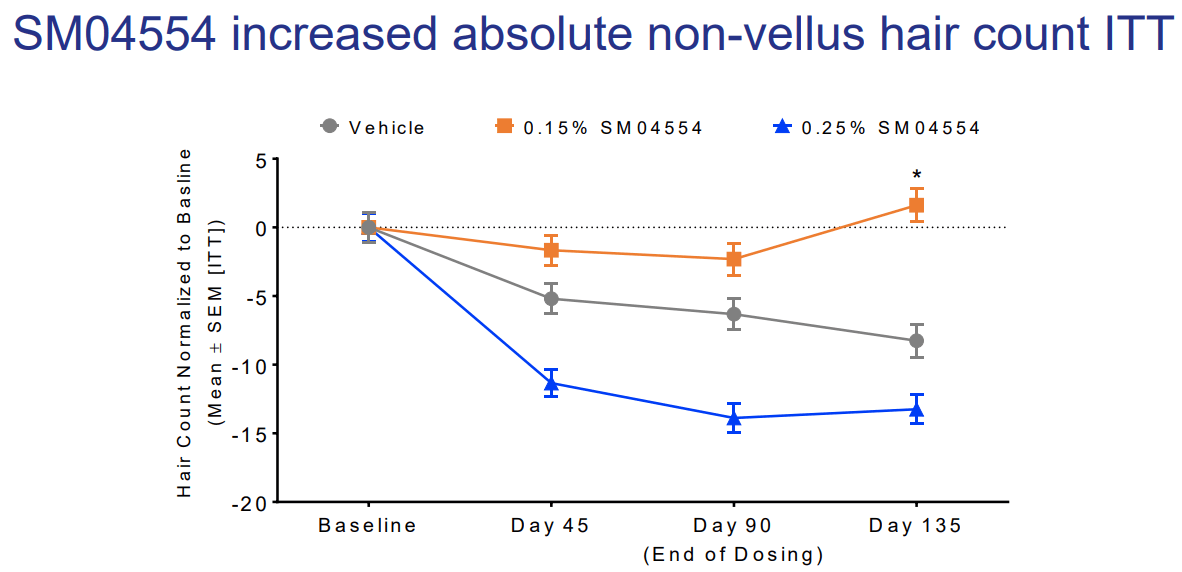
Participants received the intervention for 90 days, after which there was a follow-up 45 days later. What strikes me is that the 0.25 % solution did so much worse than the 0.15 % solution, and the 0.15 % solution also seemingly only started working after dosing was finished. (Perhaps there’s some initial shedding with this type of treatment?) Either way, the results don’t really blow me away. And I suspect the phase III trial didn’t turn out that well either. If you use the Wayback Machine to have a look at the website of Biosplice Therapeutics, you can see SM04554 still being listed on it in August 2021. If you look today, it’s gone and not present anymore on their pipeline page either. Did they give up on the drug?
Where did SM04554 go?
All in all, Wnt signaling pathway modulators hold promise, but we might need to wait a little longer before we see the first one being approved by the FDA.
Prostaglandins
Prostaglandins have surfaced as important regulators of the hair follicle cycle (as some drugs based on it, which I cover later, were found to lead to localized hair growth/hypertrichosis). They’re synthesized from the fatty acid arachidonic acid. The prostaglandin D2 (PGD2) in particular is viewed as playing a causal role in the inhibition of hair growth in androgenetic alopecia [15]. PGD2 is the product of a reaction catalyzed by the enzyme prostaglandin D2 synthase (PTGDS), for which prostaglandin H2 (PGH2) is its substrate. PGH2 is synthesized directly from arachidonic acid, a reaction catalyzed by a cyclooxygenase (COX) enzyme. So in a nutshell:
Arachidonic acid (COX)-> PGH2 (PTGDS)-> PGD2
Conversely, prostaglandin F2α (PGF2α) and prostaglandin E2 (PGE2) stimulate hair growth [16]. Both PGF2α and PGE2 are also derived from PGH2. The first synthesized by PGF2α synthase and the latter by PGE2 synthase.
The pharmaceutical industry has also explored this area of research for treatment of androgenetic alopecia. Some drugs that have been developed are a topical bimatoprost (a PGE2 analogue) and a topical latanoprost (a PGF2α analogue) solution. Both drugs were originally used to treat ocular hypertension or glaucoma, as they lower ocular pressure. But they were serendipitously found to lead to eyelash hair growth (hypertrichosis). Some small-scale clinical trials have evaluated their effects and they seem promising [17, 18].
Another drug is setipiprant, which acts as a selective prostaglandin D2 receptor antagonist. The drug is currently under investigation by Allergan Aesthetics, and in October 2021 the results of a phase 2 trial got published [19]. Participants received oral setipiprant twice daily (2x 1 g), 1 mg finasteride once daily, or a placebo, for 24 weeks. Unfortunately, however, it didn’t do any better than placebo at all.
I think we’ll hear some more of (topical) prostaglandins in the future, or drugs that might inhibit the production of PGD2 by inhibiting the enzyme PTGDS (or drugs that stimulate production of PGF2α or PGE2 by stimulating the respective enzymes that synthesize those).
References
- Rosette, Caridad, et al. “Cortexolone 17α-propionate (clascoterone) is an androgen receptor antagonist in dermal papilla cells in vitro.” Journal of drugs in dermatology: JDD 18.2 (2019): 197-201.
- Celasco, Giuseppe, et al. “Biological profile of cortexolone 17a-propionate (CB-03-01), a new topical and peripherally selective androgen antagonist.” Arzneimittelforschung 54.12 (2004): 881-886.
- Dhillon, Sohita. “Clascoterone: first approval.” Drugs (2020): 1-6.
- Mazzetti, Alessandro, et al. “Pharmacokinetic profile, safety, and tolerability of clascoterone (cortexolone 17-alpha propionate, CB-03-01) topical cream, 1% in subjects with acne vulgaris: an open-label phase 2a study.” Journal of drugs in dermatology: JDD 18.6 (2019): 563-563.
- Battmann, T., et al. “RU 58841, a new specific topical antiandrogen: a candidate of choice for the treatment of acne, androgenetic alopecia and hirsutism.” The Journal of Steroid Biochemistry and Molecular Biology 48.1 (1994): 55-60.
- Uno, H., et al. “Follicular regrowth with 5 α-reductase inhibitor (finasteride) or androgen receptor blocker (RU58841) in the bald scalp of the stumptailed macaque.” Journal of Investigative Dermatology 4.104 (1995): 658.
- De Brouwer, B., et al. “A controlled study of the effects of RU58841, a non‐steroidal antiandrogen, on human hair production by balding scalp grafts maintained on testosterone‐conditioned nude mice.” British Journal of Dermatology 137.5 (1997): 699-702.
- Poulos, Georgann A., and Paradi Mirmirani. “Investigational medications in the treatment of alopecia.” Expert opinion on investigational drugs 14.2 (2005): 177-184.
- Dhurat, Rachita, and M. S. Sukesh. “Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective.” Journal of cutaneous and aesthetic surgery 7.4 (2014): 189.
- Alves, Rubina, and Ramon Grimalt. “A review of platelet-rich plasma: history, biology, mechanism of action, and classification.” Skin appendage disorders 4.1 (2018): 18-24.
- Evans, Adam G., et al. “Platelet-rich plasma as a therapy for androgenic alopecia: a systematic review and meta-analysis.” Journal of Dermatological Treatment (2020): 1-14.
- Beaudoin, Gerard MJ, et al. “Hairless triggers reactivation of hair growth by promoting Wnt signaling.” Proceedings of the National Academy of Sciences 102.41 (2005): 14653-14658.
- Lei, Ming-Xing, Cheng-Ming Chuong, and Randall B. Widelitz. “Tuning Wnt signals for more or fewer hairs.” Journal of Investigative Dermatology 133.1 (2013): 7-9.
- Clevers, Hans, and Roel Nusse. “Wnt/β-catenin signaling and disease.” Cell 149.6 (2012): 1192-1205.
- Garza, Luis A., et al. “Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia.” Science translational medicine 4.126 (2012): 126ra34-126ra34.
- Johnstone, Murray A., and Daniel M. Albert. “Prostaglandin-induced hair growth.” Survey of ophthalmology 47 (2002): S185-S202.
- Blume-Peytavi, Ulrike, et al. “A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia.” Journal of the American Academy of Dermatology 66.5 (2012): 794-800.
- Barrón-Hernández, Yevher Lorena, and Antonella Tosti. “Bimatoprost for the treatment of eyelash, eyebrow and scalp alopecia.” Expert opinion on investigational drugs 26.4 (2017): 515-522.
- DuBois, Janet, et al. “Setipiprant for Androgenetic Alopecia in Males: Results from a Randomized, Double-Blind, Placebo-Controlled Phase 2a Trial.” Clinical, Cosmetic and Investigational Dermatology 14 (2021): 1507.
About the author
Peter Bond is a scientific author with publications on anabolic steroids, the regulation of an important molecular pathway of muscle growth (mTORC1), and the dietary supplement phosphatidic acid. He is the author of several books in Dutch and English, including Book on Steroids and Bond's Dietary Supplements.

Leave a Reply
You must be logged in to post a comment.