
Table of contents

Highlights
- Aromatizes to 17α-ME (17α-methylestradiol), a potent estrogen not detectable by either sensitive/ultrasensitive estradiol (E2) or estrone (E1) assays; 5β-reducible
- Decreases ACTH to exert its antiglucocorticoid effects
- Particularly hypertensive, and
- Potently suppressive
Introduction
Metandienone (Methandienone; Methandrostenolone; Methylandrostanolone; 17α-methyl-17β-hydroxy-Δ1,4-androstadien-3-one; 1,2-dehydro-17α-methyltestosterone; Dehydromethyltestosterone; delta-1-17 methyltestosterone; Ciba®, manufacturer, 2.5 & 5 mg tablets); Anabolin®; Dbol; herein, Dianabol) is a 1,4-diene, or a member of the class of 3-keto-androsta-1,4-dienes. Dianabol is formed by introduction of a C-1,2 (1-ene; Δ¹) double bond to methyltestosterone, is aromatizable, and 5β-reducible. [1]
Aromatization
Dianabol aromatizes to 17α-methylestradiol (17α-ME), not to be confused with 7α-ME, MENT’s aromatic product. [2] Dianabol’s 17α-ME is significantly more potent than estradiol (E2) at activating the estrogen receptor α (ER-α) that is associated with estrogenic effects (e.g., fluid retention or edema, gynecomastia). [3]

Efficacy
Determined by measuring the effect, e.g., breast cell growth, or a proxy thereof, e.g., relative light units in a luciferase bioassay. The EC50 or half maximal effective concentration is defined as the concentration at which 50% of the maximal effect (e.g., growth) occurs such that a lower EC50 indicates greater estrogenic potency.
| Estrogen | EC50 |
| 17α-ME | 12.9 ± 3.1 pM |
| E2 | 17.6 ± 7.6 pM |
[3-1]
Dianabol’s aromatic product, 17α-ME’s estrogenic potency is therefore approximately 35% greater than E2’s. This is on par with the steric hindrance posed by Dianabol’s 17α-CH₃ attachment, conceived as pulling down the rate of 17α-ME production via aromatase in vivo by 35% vs. that of testosterone vis-à-vis E2. [3-2]. In one passage that pertains to 17α-ME’s metabolism in men, “[i]t is conceivable that [17α-ME] is broken down to a much greater extent than, for example, the three classic estrogens” – or, in essence, rapidly metabolized. [2-1]
Real-world factors that frustrate positing a per-mg equivalence between Dianabol and testosterone’s relative aromatic potencies include methodological issues, indeed not rarely outright methodological mistakes, from 1960s era research about 17α-ME. [4] Ideally, data from human trials would encompass its metabolic clearance rate, the identities and effects of its metabolites, including effects on the endogenous estrogen fractions (estrone, estradiol, and estriol; E1, E2, and E2, respectively). [2-2] German researchers measured an apparent knock-on effect that 17α-ME seemed to have on E3, seemingly increasing it. [2-3] Despite a 1961 finding that certain 17α-methylated 19-nor compounds increased E3, these researchers believed that this apparent effect on E3 was not so, but rather due to an impurity masquerading as E3. [5] At the root of the obscurity that surrounds 17α-ME is the antiquated – even by contemporaneous standards – 1950s-era methods used: paper chromatography and Kober reactions using ether and benzene. [6] Breuer and Schikowski concluded their discussion about 17α-ME’s metabolism by remarking, “[e]ven if one assumes that the 17α-methyl compounds are aromatized to the same extent as testosterone, namely to about 0.4-0.6% (Breuer 1962 b), the amount of [17α-ME] produced could not be detected using the methods used here.” [2-4]

Aromatizes to a Potent Synthetic Estrogen, Not Detected by Bloodwork
- Dianabol produces 17α-ME by aromatization, that does not show up on bloodwork as E2, E1, or anything else you’re likely to find among commercial biomarkers
- Dianabol is intermediate in its estrogenic per-mg potency between MENT and testosterone.
Glucocorticoid Modulation
Effects of Dianabol on urinary excretion of 17-hydroxycorticosteroids (17-OHCS), metabolites of cortisol secreted by the adrenal gland
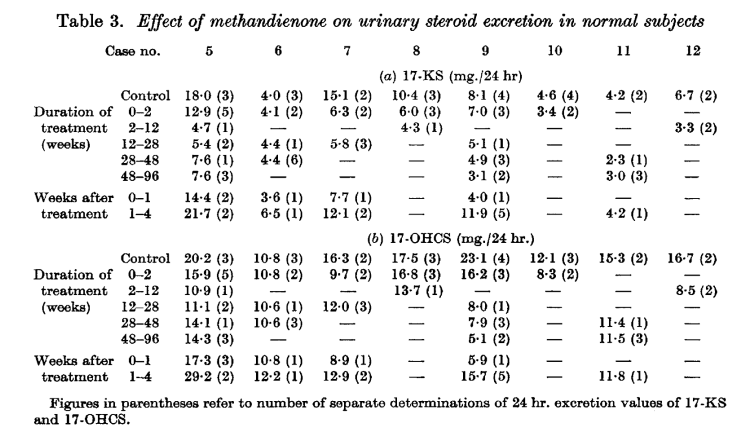
Dianabol “tamps down” the hypothalamo-pituitary-adrenal (HPA) axis at a high level, modulating glucocorticoids by lowering the synthesis and/or secretion of adrenocorticotropic hormone (ACTH). [7] Dianabol’s suppression of ACTH is apparent by the tenth day, and after cessation, ACTH returns to normal or slightly above-normal levels within 1 – 4 weeks. [7-1] This suppression of ACTH particularly affects cortisol: the largest decrease to fractions of 17-ketosteroids was to the 11-oxygenated fractions (e.g., 11-ketoaetiocholanolone). [7-2] This inhibition of the synthesis and/or secretion of endogenous ACTH could occur at the hypothalamic or the pituitary level. [7-3] There are, further, effects on the adrenal androgen pool, that can have an effect on mood, energy, and well-being.

Blocks ACTH Synthesis or Secretion, Decreasing Cortisol
- Dianabol reduces cortisol by suppressing ACTH secretion from the pituitary
Hypertension
In data from well-trained strength/power athletes (weightlifters), Dianabol’s increasing blood pressure and stroke index was apparent. [8] [9] At doses of 10 mg/day to 25 mg/day, Dianabol increased systolic blood pressure from just over 120 mmHg to 140 mmHg (significant), with no statistically significant effect on the diastolic pressure. [8-1]. At 15 mg/day, Dianabol increased stroke index, or the volume of blood the left ventricle ejects per beat [per body-surface area], by 20%. [9-1].

Effects of Dianabol on Weightlifting Performance
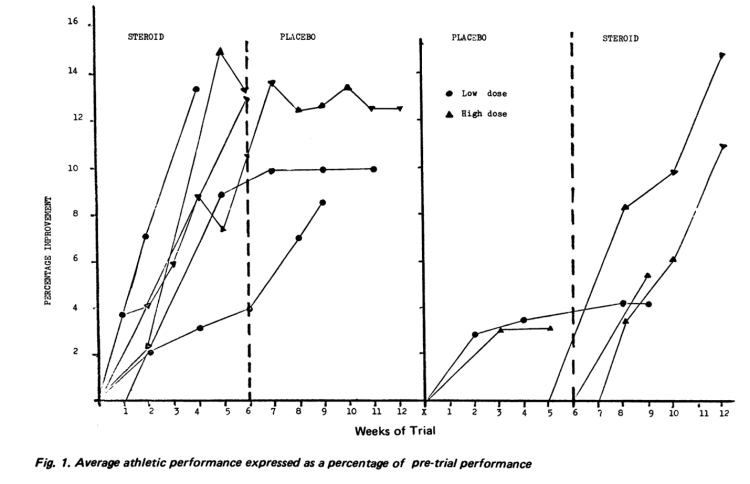

Increases Systolic Blood Pressure
- This increase to systolic blood pressure is related to increased stroke index and probably to blood volume
Antigonadotropic
Dianabol is most potently suppressive of endogenous testosterone (T), luteinizing hormone (LH), and follicle-stimulating hormone (FSH). [10] At a low-moderate dose of 15 mg daily for 2 months, plasma testosterone was reduced by 69%, LH by 53%, and FSH by 47%. [10-1] This suppression of endogenous testosterone synthesis and secretion by a mere 15 mg Dianabol is on par with 150 mg of Anadrol (-59% testosterone, -52% LH, and -39% FSH) taken for twice as long (16 weeks). [11]

Shutdown of Natural Testosterone is Rapid and Potent
- At a low-moderate dose of 15 mg, Dianabol suppresses endogenous T, LH, and FSH to the same degree within 2 months as ten-times that amount of Anadrol (150 mg, 16 weeks)
Conclusion

Summary
Dianabol’s reputation as a volumizing steroid, conducive to a full “3-D look,” but also having a proneness to cause bloating or “spilling-over,” naturally derives from its aromatization to a potent synthetic estrogen, 17α-ME. Dianabol, rather than a novice-friendly drug, is quite the opposite, since it requires careful management of estrogens while effectively running blind due to the undetectability of 17α-ME by standard assays (e.g., estradiol, estrone, or estriol). Moreover, 17α-ME is not well described. Its utility in rapidly repleting estrogens when faced with “crashed E2” symptoms is clear, however. It is easy to infer that 17α-ME is rapidly metabolized and quite powerful.
Dianabol’s modulation of glucocorticoids, by suppressing adrenocorticotropic hormone (ACTH), results primarily in decreased cortisol.
Dianabol has striking hemodynamic effects, increasing the blood volume ejected by the left ventricle, increasing stroke index and systolic blood pressure especially.
Among the 17α-alkylated androgens, Dianabol stands out as one with potent suppressive effects on endogenous testosterone, while the remainder are relatively nonsuppressive by comparison.
References
- Schänzer, W. “Metabolism of Anabolic Androgenic Steroids.” Clinical Chemistry, vol. 42, no. 7, July 1996, pp. 1001–20.
- Breuer, H., and U. Schikowski. [Studies on the Metabolism of 17α-Methyl-17β-Estradiol in Men]. Translated by Cormac Mannion, Acta Endocrinologica, 1963.
- Attardi, Barbara J., et al. “Dimethandrolone (7α,11β-Dimethyl-19-Nortestosterone) and 11β-Methyl-19-Nortestosterone Are Not Converted to Aromatic A-Ring Products in the Presence of Recombinant Human Aromatase.” The Journal of Steroid Biochemistry and Molecular Biology, vol. 110, no. 3–5, June 2008, pp. 214–22. DOI.org (Crossref), https://doi.org/10.1016/j.jsbmb.2007.11.009.
- Lutzmann, Ludger, and Erich Gerhards. [On the metabolism of Δ1-17α-Methyltestosterone (Dianabol)]. Translated by Cormac Mannion, Klinische Wochenschrift, Sept. 1961.
- Dimick, Dean F., et al. “A Comparative Study of the Metabolic Fate of Testosterone, 17α-Methyl-Testosterone, 19-Nor-Testosterone, 7α-Methyl-19-Nor-Testosterone and 17α-Methyl-Estr-5(10)-Ene-17β-Ol-3-One in Normal Males.” Clinica Chimica Acta, vol. 6, no. 1, Jan. 1961, pp. 63–71. DOI.org (Crossref), https://doi.org/10.1016/0009-8981(61)90037-7.
- Brown, J. B., et al. “An Additional Purification Step for a Method for Estimating Oestriol, Oestrone and Oestradiol-17β in Human Urine.” Journal of Endocrinology, vol. 16, no. 1, Nov. 1957, pp. 49–56. DOI.org (Crossref), https://doi.org/10.1677/joe.0.0160049.
- Wynn, V., et al. “Effect of an Anabolic Steroid (Methandienone) on Pituitary-Adrenal Function in the Human.” Journal of Endocrinology, vol. 25, no. 2, Oct. 1962, pp. 199–209. DOI.org (Crossref), https://doi.org/10.1677/joe.0.0250199.
- Freed, D. L., and A. J. Banks. “A Double-Blind Crossover Trial of Methandienone (Dianabol, CIBA) in Moderate Dosage on Highly Trained Experienced Athletes.” British Journal of Sports Medicine, vol. 9, no. 2, July 1975, pp. 78–81. DOI.org (Crossref), https://doi.org/10.1136/bjsm.9.2.78.
- Holma, P. “Effect of an Anabolic Steroid (Metandienone) on Central and Peripheral Blood Flow in Well-Trained Male Athletes.” Annals of Clinical Research, vol. 9, no. 4, Aug. 1977, pp. 215–21.
- Holma, Pentti, and Herman Adlercreutz. “Effect of an Anabolic Steroid (Metandienon) on Plasma LH, FSH, and Testosterone and on the Response to Intravenous Administration of LRH.” Acta Endocrinologica, vol. 83, no. 4, Dec. 1976, pp. 856–64. DOI.org (Crossref), https://doi.org/10.1530/acta.0.0830856.
- Hengge, Ulrich R., et al. “Double-Blind, Randomized, Placebo-Controlled Phase III Trial of Oxymetholone for the Treatment of HIV Wasting:” AIDS, vol. 17, no. 5, Mar. 2003, pp. 699–710. DOI.org (Crossref), https://doi.org/10.1097/00002030-200303280-00008.
About the author
Type-IIx is a physique coach, author, and researcher. Bolus: A Practical Guide and Reference for recombinant Human Growth Hormone Use will be his first published textbook, anticipated for release in early 2023. Ampouletude.com will be Type-IIx's base of operations for coaching services and publications. Type-IIx is proud to be a contributing writer to MesoRx, his home forum, where he is a regular poster.
Leave a Reply
You must be logged in to post a comment.