
Hi-Tech Pharmaceuticals President and CEO Jared R. Wheat, Vice President Stephen D. Smith, co-founder Tomasz Holda and Sales Associate Sergio Oliveira pleaded guilty last week to one count of conspiracy i.e “conspiring to violate federal prohibitions against mail and wire fraud and the importation and distribution of adulterated, unapproved, and mislabeled drugs.” The defendants admitted to the operation of a pharmaceutical manufacturing facility doing business as Planet Pharmacy in Belize where they produced generic versions of various prescription drugs which were sold without a prescription on the Internet (“Four “Hi-Tech Pharmaceuticals” Case Defendants Plead Guilty to Importing and Distributing “Knock-Off” Prescription Drugs,” August 18).
United States Attorney David E. Nahmias said of the pleas, These defendants set up an offshore manufacturing facility where, in unsanitary conditions, they reproduced leading pharmaceutical products for importation into the United States, all without FDA approval or licensing from the rightful patent holders. Their motive in flouting the law, violating patents and exposing their customers to unknown health risks was greed, pure and simple. I commend the FDA and the DEA for their thorough investigation in this case. The Department of Justice and these agencies will continue to work hard to protect American consumers from such fraudsters.
The U.S. Attorney did not mention that Planet Pharmacy, operating in Belize, could legally produce generic versions of drugs such as Xanax, Valium, Ambien, Vioxx, Lipitor, Zoloft, Viagra, Cialis, Anavar, Anadrol, Dianabol, Winstrol, Arimidex, Clomid, Nolvadex, etc. because of the existence of a loophole in Belize law. Planet Pharmacy believed they were legally operating as long as they did not directly market or distribute the products to residents of the United States.
Belize does not recognize USPTO world patents granted to pharmaceutical companies. Therefore, Belize does NOT legally require the approval of U.S. patent holders or the U.S. Food and Drug Administration (FDA) to manufacture generic drugs. Planet Pharmacy needed to avoid marketing and distributing the products to residents of the United States. Furthermore, they needed to only export the generics to wholesalers in other countries where there was weak or non-existent patent protection for pharmaceutical patents.
Yet, the government held Planet Pharmacy accountable for the actions of third party wholesalers who shipped generic prescriptions, including anabolic steroids, into the United States. Prosecutors asserted that Planet Pharmacy was criminally responsible for directly importing medications into the United States, in part, because they “knew” that Pmeds.com, one of their Mexican wholesalers, was shipping parcels to residents of the United States.
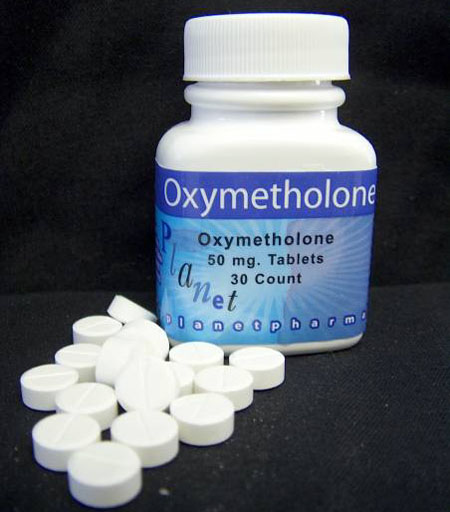
A DHL shipping receipt was submitted by prosecutors as further proof that Planet Pharmacy imported generic drugs into the United States. The DHL receipt stated that “empty bottles” were sent to William Llewellyn, author of Anabolics 2007, in Florida. As everyone in the industry knows, Llewellyn actively requests empty steroid bottles and vials so that he can include photographs of them for his anabolic steroid reference book. Photographs of Planet Pharmacy Oxandrolone, Dianabol and Stanozolol bottles are all included in Appendix E of Anabolics 2007.
FDA Office of Criminal Investigations Special Agent In Charge David Bourne said, As in this conviction, the FDA’s Office of Criminal Investigations actively pursues those who deceive the public by manufacturing and selling unapproved and unregulated medications which may pose risks to the health of consumers. We are committed to investigating and preventing those who use trickery and deceit to illegally and unscrupulously sell medications over the internet at the expense of the public health.
Planet Pharmacy manufactured several oral anabolic steroids and ancillary medications; all reports from San Rafael Chemical Services (SRCS) showed these medications to be free of impurities and accurately dosed, nonsterile conditions notwithstanding.
Practically all medications subject to importation by U.S. residents are either not approved by the FDA or not regulated by the FDA; importing medications without a valid medical prescription is also a violation of federal law. As such, the government considers any such purchase of an inexpensive generic medication without a prescription an unacceptable risk to the health of consumers. This is not unique to Planet Pharmacy.
- Federal law prohibits the importation of an FDA-approved pharmaceuticals that is manufactured within the United States. (21 U.S.C. § 381(d)(1))
- Federal law prohibits the importation of pharmaceuticals that are not approved by the FDA. (21 U.S.C. § 355)
- Federal law prohibits the importation of an FDA-approved pharmaceutical produced by a foreign manufacturer that does not have FDA approval for that drug. (21 U.S.C. § 355)
- Federal law prohibits the importation of an FDA-approved pharmaceutical produced by a foreign manufacturer even when that firm has FDA approval if the version produced for foreign markets does not meet the various requirements of U.S. approval in all respects. (21 U.S.C. § 355)
- Federal law prohibits the importation of pharmaceuticals that do not meet all U.S. labelling requirements and bears FDA approved labelling. (21 U.S.C. § 353(b)(2)
- Federal law prohibits the importation of pharmaceuticals that are dispensed without a valid prescription. (21 U.S.C. § 353(b)(1))
Prior to the government’s lawsuit against Hi-Tech, the pharmaceutical giant Pfizer filed a complaint with the United States International Trade Commission to stop Planet Pharmacy from manufacturing low-cost generic prescription versions of Pfizer’s expensive brand name drugs. This was unsuccessful. In addition, the U.S. Embassy in Belize filed a complaint with the Belize Intellectual Property Office (BELIPO). This was also unsuccessful.
The U.S. DEA and FDA subsequently used their power and influence to shutdown Planet Pharmacy in a legally questionable police action in conjunction with Belizean authorities. This was followed by over-reaching, trumped up criminal charges in a 45-count federal indictment. The defendants in the case ultimately pleaded guilty to a single count of conspiracy after a protracted legal battle. The most serious, salacious and sensational government allegations, which the defendants called “false,” “far-fetched,” “baseless” and “highly reckless” were dropped by federal prosecutors; the Court had already acknowledged in 2007 that “[the Government’s] evidence indicative of Jared Wheat’s involvement in the charged criminal activities is not as strong as the Court previously thought.” The Government also dropped allegations involving the illegal importation and distribution of anabolic steroids (“From diet supplements to illegal Net pharmacy,” August 19).
Prosecutors dropped the most serious charge against Wheat, which accused him of engaging in a continuing criminal enterprise, in the plea negotiations. That charge could have landed Wheat in prison for a minimum of 20 years and resulted in the forfeiture of his company.
Prosecutors also backed away from earlier assertions that Hi-Tech’s successful lines of herbal dietary supplements ” they are carried by many major U.S. retailers as well as thousands of convenience stores ” had been spiked with ephedrine alkaloids even after the U.S. Food and Drug Administration banned their use. The FDA banned the substance ” the active agent in the ephedra plant ” in 2004 after finding that it presented an unreasonable risk of illness or injury.
Also missing from the final plea deal was any reference to the prosecution’s previous contention that Wheat, Smith and Holda conspired to murder a U.S. Food and Drug Administration agent and blackmail a former assistant U.S. attorney general. That sensational allegation, based on information provided by confidential informants, surfaced in court documents filed in March 2006 by prosecutors in response to Wheat’s bid to be released on bond.
The making of laws has famously been compared to the making of sausage where one can enjoy the results but only if they do not witness the ugly process. The government actions in the case against Hi-Tech provides a strong argument that sometimes the alleged enforcement of laws is even uglier. The dubious use of RICO laws (continuing criminal enterprise), specious allegations of blackmail and murder-assassination plots, the reckless intimidation of a witness to the point of suicide were all tactics used to shutdown a small Belize pharmaceutical manufacturing company that produced extremely low cost generic medications.
Certainly, there are victims in this case. Perhaps the consumers, many likely without health insurance, who purchase cheap generic prescription medications from Planet Pharmacy were not the real victims.
In the government’s alleged effort to protect the public health they displayed a reckless disregard for the health of Jessica Holda, the spouse of one the defendants who pleaded guilty in this case. Prosecutors intimidated her and threatened her with prosecution if she did not testify against her husband in the case; federal prosecutors even used the Georgia Department of Family and Children Services in an attempt to remove Tom and Jessica Holda’s 18 month daughter from their custody. The government felt they could scare Jessica Holda into testifying due to her fragile emotional state and severe anxiety disorder. The outcome was decidedly different. Jessica Holda took her own life on February 19, 2007. RIP.
The government’s paternalistic attempts to protect American consumers from cheap generic medications overseas regrettably has its own collateral damage.
About the author
Millard writes about anabolic steroids and performance enhancing drugs and their use and impact in sport and society. He discusses the medical and non-medical uses of anabolic-androgenic steroids while advocating a harm reduction approach to steroid education.

Leave a Reply
You must be logged in to post a comment.