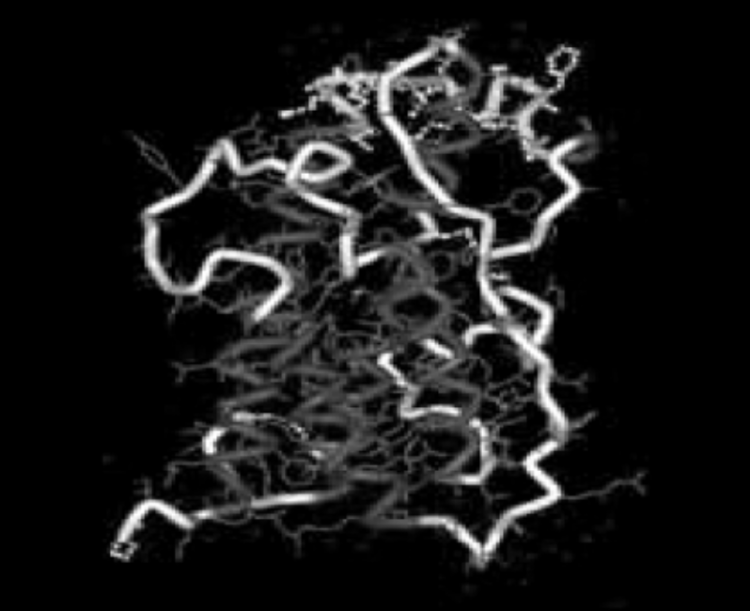
Circulating GH is largely bound with carrier proteins referred to as GHBPs, or growth hormone binding proteins. These carrier proteins are essentially a soluble and truncated form of the extracellular domain of the GHR – mobile circulating GHRs which are not located within cellular membranes, if you will [103]. GH in circulation can also exist as free or unbound and the ratio of bound versus unbound is dependent upon the pulsatile pattern of its secretion [104]. Circulating GH complexes in humans can be comprised of one of two distinct GH molecules (22-kDa and 20-kDa), with roughly 90% being the 22-kDa molecule despite early estimates putting that number much lower [105-106]. Fun fact, modern indirect GH doping test methodologies can actually leverage the real-time ratios of circulating GH molecules within an athlete’s system to determine if someone has used rHGH in the past 24-36 hours prior to the test. The growth hormone molecule, in its correct 22-kDa form, is pictured below [107]:
Ultimately, this circulating GH binds with GHRs, which are class one cytokine receptors expressed in numerous cell-type membranes throughout the body [108-110]. The cellular surface levels, or receptor density, of these GHRs are the primary determinant of GH’s binding affinity to cells. GHR translocation, or the receptor relocating from a cell’s nucleus to its external membrane, is directly inhibited by IGF-1 – which is one of many feedback mechanisms that exist between these tightly related hormones. By inhibiting GHR translocation, IGF-1 directly contributes to lower the responsiveness of these cells to an external GH stimulus [111].
A deep-dive into tyrosine kinase activation, downstream signaling pathways, and gene expression is a bit beyond the scope of this article. So instead we’ll just stick our toes in the water and get them a little wet. I feel that we must touch on some of the high points of intracellular signaling to truly understand the underpinnings of the hypertrophy process, and why there are both compound synergies and potential optimized strategies for maximizing the process.
As mentioned already, GHRs exist on cellular membranes and they exist as preformed and inactive homodimers. This is really just a fancy way of saying the GHR has two identical protein receptor dimers, and these homodimers are always going to be coupled to JAK2 when devoid of enzymatic activity. This coupling to JAK2 causes an overall inhibitory action on the receptor [112-113]. In other words, the GHR lays dormant until it is activated as part of the GH/GHR binding process. When a GH molecule binds to the GHR, a structural change occurs within the GHR that results in actual movement of the receptor’s intracellular domains apart from one another. This relieves that inhibitory action of the JAK2 molecules and allows them to activate one another [114-116].
Next, one GH molecule binds sequentially to one of the two GHR homodimers, and the completion of this binding process facilitates interactions with the second homodimer. After this occurs, the intracellular domains of this newly formed GHR dimer undergo an actual rotation. Rotating the new GHR dimer allows the kinase domains of JAK2 to be in contact with one another, allowing them to transactivate and each subsequently binds to one JAK2 molecule [117-118]. Each JAK2 molecule will then perform cross-phosphorylation (activation) of tyrosine residues, and it is these residues which form “docking sites” for many of the different signaling molecules that make up the downstream signaling pathways, and ultimately lead to gene expression [114,118-120]. One of the more important downstream pathways for our purposes is the JAK-STAT pathway. This pathway is vital for both the hepatic transcription of IGF-1 by GH as well as many of the GH-mediated anabolic processes within skeletal muscle tissue. The vast majority of this complicated section was largely just background, so we could simply get to this last point.
Okay, my apologies, as I suppose it was our entire leg that just got wet and not only our toes. In any event, let’s take time to exhale for a moment while we move away from signaling pathways and go back to some higher level information for a bit…
A Primer on IGF-1
We’ve previously gone over both the history and timeline by which GH and IGF-1 were discovered. However, I’d still like to cover some additional ground on the insulin-like growth factor family members to more firmly establish what they are, in addition to their roles in the hypertrophy process. The IGFs are a family of peptides, largely GH-dependent, who mediate many of the growth promoting actions that GH has [121]. The liver is chiefly responsible for all endocrine IGF-1 production, with around 75% being hepatically produced under the regulation of GH [83,122-123]. This assumes there are both sufficient dietary intake and elevated portal insulin levels [124-125]. Autocrine IGF-1 synthesis is also regulated by GH, in addition to other tissue-dependent autocrine factors [126-128].
Leave a Reply
You must be logged in to post a comment.