Hello,
sharing a discourse between me and a manufacturer ("Man") regarding HCG testing for the community.
In response to my report and raw data at the beginning of the discussion:
Man:
Hello,
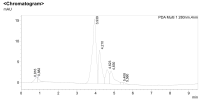
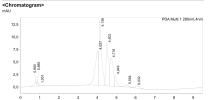
Yes I have it now. A question, do you calculate only the peak which is marked with a letter V on 3rd line below the HPLC graph? Also why you dont include mg and only IU is included?
Jano:
Hello,
the peaks are respective subunits of HCG. 45 amino acid beta-subunit that is unique to hCG and a 92 amino acid alpha-subunit.
You always calculate the IU from the subunit which is lower in proportion. In this case it was the beta subunit.
We don't include MG result because it's mostly useless for our clients, who are only used to international units. Your result is very roughly equivalent to 124 micrograms of HCG.
Hope I helped.
Man:
Thanks for the reply, regarding HCG bioactivity could you please explain and send a link to the HCG, LH bioactivity measurement using HPLC, as we are a bit surprised by your method measuring bioactivity of hormones using HPLC. As far as it is known, the HCG molecule has a variety of isoforms, showing different potency in bioactivity.
Human derived HCG potency is different not only to mammalian cell derived hcg potency or yeast cell derived with human like glycosylation potency, there is also a big variance of potency from batch to batch.
The only certified method of measuring HCG, LH hormone is mouse Leydig cell bioassay, and measurement of testosterone production when incubated with hormone, you can find it on the internet https://d-nb.info/1125996250/34, could you please supply the proof of validity of
your HPLC method of measuring bioactivity.
As I mentioned in my previous email, we are direct manufacturer of this HCG, and many of our clients send our HCG vials to test at your place, that’s why accurate testing is very important in this case, because we are selling this HCG at large wholesale amounts to many known clients in EU. Our HCG is recombinantly derived from yeast, not from female urine and not from Chinese hamster ovarian cells.
Many thanks
Jano:
Hello,
you are correct that HPLC measurement of HCG is not listed in any pharmacopoeia and that bioassay is the method suggested by certifying authorities, however, we do not conduct bioassays and our HPLC method for the HCG is standard only for our company.
Our HPLC method is simply comparing the amounts of human identical alpha and beta subunits present with the standard that we have, we disregard any isoforms.
Hope this helps.
Man:
Hello,
So basically this means that your HPLC measurement of HCG can't be treated as accurate and your method isn't suggested by any certifying authorities.
Jano:
>So basically this means that your HPLC measurement of HCG can't be treated as accurate
That is not what it means.
>your method isn't suggested by any certifying authorities.
That is correct.
And there is no HPLC method for anabolic steroid analysis suggested by any certifying authorities and yet it's 90% of our business.
Man:
Hello,
I want to get back to you regarding your HCG testing, I spoke to other HCG producers who produce it using chinese HCG raw material, also to some other big sellers of HCG who import HCG vials, they all get same lower results than the expected 5000IU, and results range in 3000-3500IU which is a very low result.
As an example I sent vial from same batch, which you tested a bit above 3000IU to Analiza Bialek lab, and they showed correct results of nearly 6000IU. Check attachment.
You should really recheck how you test HCG and what standard you use, because this problem I see is not only with my production HCG but its mainstream problem which you have which affects HCG sales, with your current testing everyone has to add 10000IU so you would show 5000IU in your testing.
I am not pointing to your oils testing or your HGH testing, I am specifically saying about your HCG testing.
In a few weeks I will start bioassay testing for my HCG vials and I will be able to return you with even more specific results and compare with the results you get.
Regards.


Jano:
How much did that analysis cost, please?
Will get to your email in full length tomorrow or so.
Thank you
Man:
Hello,
175 EURO, yes I look forward to your update, thanks.
Regards.
Jano:
Thank you.
Regarding Analyza Bialek:
Could you also please tell me, how much does the HCG standard cost? Because the cheapest I have found is 280 Euro and that's not even the reference standard my friend.
Can they supply the documentation for the standard?
As HCG standards are nowhere near 175 Euro, so I am really interested in what Analyza Bialek is referring to when they talk about HCG reference.
Or do you believe they did the analysis at a loss or do you believe they test HCG so much that they can spread the costs over several samples a month, which is about the maximum longevity of such a standard?
Because if they tested multiple HCG samples every month, I'd assume I would have seen something about that. And I did not.
One more thing, as you surely do know, HCG is a mixture of alpha and beta subunits.
Thus on a proper chromatogram you ought to see at least two peaks. On the chromatogram supplied by that company you see one.
A laboratory which is confident enough to release quantitative data on an analyte they cannot even properly separate is... well, let's just say that it would be an insult for me to be compared to such a standard.
I honestly do believe, from the data available, that their IU number is made up.
----------------------------
Regarding the issue with my testing:
Bioassay, which I assume you mean immunoassay, ELISA sandwich usually, is actually less specific than properly set up HPLC.
For example, when measuring estradiol, trenbolone causes interference on immunoassays, because of similar binding points.
Now, if you have HCG, but one or both of the subunits are slightly different - damaged, degraded or just synthesis byproducts or isoforms in case of biologically derived sample, they will still react with ELISA and they will, indeed,, usually elicit a biological response in human body - which is what you and clients are looking for.
But from the chemical sense of things an HCG that is damaged/isoform is not HCG. I am measuring just HCG subunits that are exact or extremely similar to the point of not being discernible on HPLC from the HCG as per definition.
Thus the result that is usually lower than ELISA tests.
If there is anything that is not clear or if you have any objections, please, let me know.
Man:
Hello,
Well, I dont know how much HCG samples they test per month and how this lab operates its non of my business.
To make it short, HPLC isnt approved testing method for HCG vials. The approved method I am talking about is this:
Comparison of human chorionic gonadotropin measurements by radioimmunoassay and in vitro bioassay - PubMed
We will start performing this method on our production HCG batches in around 4 weeks, when we can recheck and compare our results with your HPLC results and can discuss further. But as I stated in my previous email, most of the producers/sellers I know, have problem with your HCG results lately showing much less than it really is inside and this affects sales because end users believe in your test results. This is mainstream issue, not coming only from my HCG.
Regards.
Jano:
Well, you used their work as an argument why I am wrong, so you sort of made it my business, so I did indeed feel the necessity to address that.
Radioimmunoassays are not really used anymore and the article you have linked in from 1985.
In fact, US pharmacopoeia from 2005 - thus 30 years newer - suggests, or suggested assaying HCG via injecting it into female rats and then measuring the mass of their uteri - USP Monographs: Chorionic Gonadotropin
I believe I have explained well enough why different methods can yield different results and I am in fact very honored to be trusted by the community.
I believe this discussion can help explain to the community why the results indeed are different. Would you, perhaps, give me a permission to share the educative parts of our discussion that are anonymized with the community?
Thank you.
Man:
Hello,
Regarding the HCG biological activity measurement using HPLC:
The point is not whether the method which we sent to you is old, the point is what method is used to assess the biological activity of HCG, be it: using rats uterus or using Leyding cells and expression of testosterone or cAMP, these are all valid methods of assessing bioactivity, with available publications, usage in pharmacopeia etc., HPLC is not one of these methods and there is very good reason why it is not used. If you think otherwise, that’s fine, as long as you can supply your used methods of testing which have publications and are widely accepted, and we can compare the results and have a starting point from where to discuss further.
Regarding sharing our conversation to the community, of course, but only after we finish it, we are still in the middle of it.
Kind regards.
Jano:
Hello,
saying that HPLC isn't a valid method, but having posted an HPLC analysis of HCG to support your argument just a few emails ago is not really something that looks like a good faith argument here.
Regarding RPHPLC there are multiple publications that I was able to check, such as: Analysis of the human chorionic gonadotropin protein at the intact level by HILIC-MS and comparison with RPLC-MS - PubMed
Hope this helps.
Man:
Hello,
Regarding the publication you sent:
I am not sure if you have read the publication which you sent, there is not any mention about bioactivity measurement using HPLC, if you would read that publication you’ll see why exactly no-one is using HPLC for that purpose, how many isoforms there is, how hard just to fingerprint them etc.
And regarding HPLC results which I sent from another independent lab from same batch of HCG vials, yes their method is also not approved anywhere, I simply sent it to show results difference.
Jano:
Hello,
well, I believe that publication underlined rather well both the fact that RP-HPLC analysis is quite well possible, although, I agree with you, complicated, and that the Analyza Bialek's report is not worth the paper it is printed on if they weren't able to separate even the hCG alpha and beta subunits.
Also, as it is mentioned, the recombinant hCG glycoforms are by far not as numerous as the hCG au naturel and we don't really need to fingerprint them, as we are not really interested in the glycation details.
Although, it is entirely possible, that given we use rhCG standard, this might introduce higher error with urine derived hCG, that I admit.
No one is using HPLC methods in Pharmacopoeias because Pharmacopoeias are legacy products and they are updated with a high regard to sort of 'backwards' compatibility.
I can assure you that if the Pharmacopoeias were not updated with a high regard to 'backwards' compatibility you wouldn't be seeing tests on living animals
However, I believe it might be appropriate to add an commentary to our reports, that our reports are comparable only to our reports and that other testing methods can and will yield different results.
Man:
Hello,
Yes HPLC analysis of HCG is possible, no doubt about that, but not in order to determine HCG activity.
Regarding the rHCG vs HCG, yes you are right in that regard that it is possible to better approximate bioactivity of rHCG using HPLC than human derived HCG, but then is the question, how do you know what HCG you receive for analysis, because the biggest share in the market which you are testing is human derived , the rest share of the market is rHCG and not all from the same host, there is CHO, yeast rHCG, so in order to approximate bioactivity of rHCG you have to have that host's rHCG as your standard with known bioactivity, which anyway has to be determined using other methods.
Regarding why no-one us using HPLC method in pharmacopeia you are not correct, as HPLC would be much cheaper and easier method to determine HCG bioactivity if it was possible, everyone would use it, if it would be possible to validate it for determination of HCG bioactivity in production or testing purposes, but no-one uses it because it is not suitable method for this particular protein, due to huge variation in glycolisation pattern and that pattern has big influence to bioactivity, so one protein with the same amount of mg/ml with one glycolisation pattern can have very different bioactivity with the same amount mg/ml with different glycolisation pattern.
I would not agree that no one is using hplc in pharmacopoeia, for lot's of proteins HPLC validation is the best and most important step in determination of protein characteristics, but not all proteins are the same. And in this regard you can not measure everything using the same scheme.
Regarding testing not only protein's but other medicine in animals and humans, that is done not because it's some legacy or backward way of doing things, but there is very good reason why it is done this way, (i think the animals in our days except small rodents etc., is not used in modern world for trials), because in order to see the full spectrum of the molecules influence on the target body(system), you have to use as similar as possible body(system) for the target body(system) intended, as there is so much pathways of interaction in the body(system) that the cheapest way to repeat what is happening in the body(system) intended is to use as similar as possible body(system), for human's it's primates but nowadays it is rarely used and after initial testing on small rodents testing continues on human's, with human consent and payment accordingly.
About adding your suggested commentary on HCG test repots, yes we agree and that would make the confusion more clear which is going around right now regarding your HCG testing results.
Regards.
Jano:
>Yes HPLC analysis of HCG is possible, no doubt about that, but not in order to determine HCG activity.
Yes, for activity that's merely an estimation. But would my clients understand if I listed the amount in micrograms? No.
So I have to keep the reports simple.
>Regarding the rHCG vs HCG, yes you are right in that regard that it is possible to better approximate bioactivity of rHCG using HPLC than human derived HCG, but then is the question, how do you know what HCG you receive for analysis, because the biggest share in the market which you are testing is human derived , the rest share of the market is rHCG and not all from the same host, there is CHO, yeast rHCG, so in order to approximate bioactivity of rHCG you have to have that host's rHCG as your standard with known bioactivity, which anyway has to be determined using other methods.
For hdHCG the high amount of glycosilation patterns is observable. Regarding the rest, there's a tradeoff between keeping the testing accessible and keeping the testing perfect. I find that our margin of error is perfectly acceptable to all of our clients.
>Regarding why no-one us using HPLC method in pharmacopeia you are not correct, as HPLC would be much cheaper and easier method to determine HCG bioactivity if it was possible, everyone would use it, if it would be possible to validate it for determination of HCG bioactivity in production or testing purposes, but no-one uses it because it is not suitable method for this particular protein, due to huge variation in glycolisation pattern and that pattern has big influence to bioactivity, so one protein with the same amount of mg/ml with one glycolisation pattern can have very different bioactivity with the same amount mg/ml with different glycolisation pattern.
Correct. However I do believe that an accessible, 180 USD, however imperfect test has way more value to people than perfect 5000 USD test.
If you believe my clients are after the perfect 5000 USD test more than after an imperfect +-10/20% test for 180 USD for HCG, it is a free market and you can start the testing company focused on delivering the perfect HCG testing. I will keep on doing what I believe is the best for my clients.
>I would not agree that no one is using hplc in pharmacopoeia, for lot's of proteins HPLC validation is the best and most important step in determination of protein characteristics, but not all proteins are the same. And in this regard you can not measure everything using the same scheme.
You are right, I misconstructed the argument - I meant no one is using cutting edge HPLC methods, because of having to maintain legacy compatibility.
Naturally, I did not mean that HPLC methods are not used in Pharmacopoeias at all.
>Regarding testing not only protein's but other medicine in animals and humans, that is done not because it's some legacy or backward way of doing things, but there is very good reason why it is done this way, (i think the animals in our days except small rodents etc., is not used in modern world for trials), because in order to see the full spectrum of the molecules influence on the target body(system), you have to use as similar as possible body(system) for the target body(system) intended, as there is so much pathways of interaction in the body(system) that the cheapest way to repeat what is happening in the body(system) intended is to use as similar as possible body(system), for human's it's primates but nowadays it is rarely used and after initial testing on small rodents testing continues on human's, with human consent and payment accordingly.
However, that's more for preclinical testing or, in pharmacological settings, for introduction of manufacture, not routine analysis, which is what I'm usually doing.
Do you, actually, conduct the testing as per the pharmacopoeia?
Anyway, I will add the commentary to my HCG testing that Janoshik HCG tests are only comparable to other Janoshik HCG tests and may differ from bioactivity testing on rodents once I have the time.
Thank you for a fruitful discussion, but there is only so much time I can spend on a singular topic, so I believe we can assume our discussion to be concluded.
Have a good weekend.
Man:
Hello,
Thanks for the reply and stating all the facts.
Yes please let me know when you can add the commentary to HCG lab results, this is important for the clients and for the people who use their HCG. Can you do this week?
Also as we discussed previously, you can publish our conversation for public for them to be fully aware of HCG testing methods and accuracy of different testing methods, especially HPLC testing method for HCG.
Regards.
Jano:
Hello,
unfortunately, I cannot start with adding the commentary I mentioned to the reports this week, as the commentary is already being added to reports of HCG since about 10 days ago already
Thank you, will be doing so shortly. Hope you have a good day!
- Jano
sharing a discourse between me and a manufacturer ("Man") regarding HCG testing for the community.
In response to my report and raw data at the beginning of the discussion:
Man:
Hello,
Yes I have it now. A question, do you calculate only the peak which is marked with a letter V on 3rd line below the HPLC graph? Also why you dont include mg and only IU is included?
Jano:
Hello,
the peaks are respective subunits of HCG. 45 amino acid beta-subunit that is unique to hCG and a 92 amino acid alpha-subunit.
You always calculate the IU from the subunit which is lower in proportion. In this case it was the beta subunit.
We don't include MG result because it's mostly useless for our clients, who are only used to international units. Your result is very roughly equivalent to 124 micrograms of HCG.
Hope I helped.
Man:
Thanks for the reply, regarding HCG bioactivity could you please explain and send a link to the HCG, LH bioactivity measurement using HPLC, as we are a bit surprised by your method measuring bioactivity of hormones using HPLC. As far as it is known, the HCG molecule has a variety of isoforms, showing different potency in bioactivity.
Human derived HCG potency is different not only to mammalian cell derived hcg potency or yeast cell derived with human like glycosylation potency, there is also a big variance of potency from batch to batch.
The only certified method of measuring HCG, LH hormone is mouse Leydig cell bioassay, and measurement of testosterone production when incubated with hormone, you can find it on the internet https://d-nb.info/1125996250/34, could you please supply the proof of validity of
your HPLC method of measuring bioactivity.
As I mentioned in my previous email, we are direct manufacturer of this HCG, and many of our clients send our HCG vials to test at your place, that’s why accurate testing is very important in this case, because we are selling this HCG at large wholesale amounts to many known clients in EU. Our HCG is recombinantly derived from yeast, not from female urine and not from Chinese hamster ovarian cells.
Many thanks
Jano:
Hello,
you are correct that HPLC measurement of HCG is not listed in any pharmacopoeia and that bioassay is the method suggested by certifying authorities, however, we do not conduct bioassays and our HPLC method for the HCG is standard only for our company.
Our HPLC method is simply comparing the amounts of human identical alpha and beta subunits present with the standard that we have, we disregard any isoforms.
Hope this helps.
Man:
Hello,
So basically this means that your HPLC measurement of HCG can't be treated as accurate and your method isn't suggested by any certifying authorities.
Jano:
>So basically this means that your HPLC measurement of HCG can't be treated as accurate
That is not what it means.
>your method isn't suggested by any certifying authorities.
That is correct.
And there is no HPLC method for anabolic steroid analysis suggested by any certifying authorities and yet it's 90% of our business.
Man:
Hello,
I want to get back to you regarding your HCG testing, I spoke to other HCG producers who produce it using chinese HCG raw material, also to some other big sellers of HCG who import HCG vials, they all get same lower results than the expected 5000IU, and results range in 3000-3500IU which is a very low result.
As an example I sent vial from same batch, which you tested a bit above 3000IU to Analiza Bialek lab, and they showed correct results of nearly 6000IU. Check attachment.
You should really recheck how you test HCG and what standard you use, because this problem I see is not only with my production HCG but its mainstream problem which you have which affects HCG sales, with your current testing everyone has to add 10000IU so you would show 5000IU in your testing.
I am not pointing to your oils testing or your HGH testing, I am specifically saying about your HCG testing.
In a few weeks I will start bioassay testing for my HCG vials and I will be able to return you with even more specific results and compare with the results you get.
Regards.


Jano:
How much did that analysis cost, please?
Will get to your email in full length tomorrow or so.
Thank you
Man:
Hello,
175 EURO, yes I look forward to your update, thanks.
Regards.
Jano:
Thank you.
Regarding Analyza Bialek:
Could you also please tell me, how much does the HCG standard cost? Because the cheapest I have found is 280 Euro and that's not even the reference standard my friend.
Can they supply the documentation for the standard?
As HCG standards are nowhere near 175 Euro, so I am really interested in what Analyza Bialek is referring to when they talk about HCG reference.
Or do you believe they did the analysis at a loss or do you believe they test HCG so much that they can spread the costs over several samples a month, which is about the maximum longevity of such a standard?
Because if they tested multiple HCG samples every month, I'd assume I would have seen something about that. And I did not.
One more thing, as you surely do know, HCG is a mixture of alpha and beta subunits.
Thus on a proper chromatogram you ought to see at least two peaks. On the chromatogram supplied by that company you see one.
A laboratory which is confident enough to release quantitative data on an analyte they cannot even properly separate is... well, let's just say that it would be an insult for me to be compared to such a standard.
I honestly do believe, from the data available, that their IU number is made up.
----------------------------
Regarding the issue with my testing:
Bioassay, which I assume you mean immunoassay, ELISA sandwich usually, is actually less specific than properly set up HPLC.
For example, when measuring estradiol, trenbolone causes interference on immunoassays, because of similar binding points.
Now, if you have HCG, but one or both of the subunits are slightly different - damaged, degraded or just synthesis byproducts or isoforms in case of biologically derived sample, they will still react with ELISA and they will, indeed,, usually elicit a biological response in human body - which is what you and clients are looking for.
But from the chemical sense of things an HCG that is damaged/isoform is not HCG. I am measuring just HCG subunits that are exact or extremely similar to the point of not being discernible on HPLC from the HCG as per definition.
Thus the result that is usually lower than ELISA tests.
If there is anything that is not clear or if you have any objections, please, let me know.
Man:
Hello,
Well, I dont know how much HCG samples they test per month and how this lab operates its non of my business.
To make it short, HPLC isnt approved testing method for HCG vials. The approved method I am talking about is this:
Comparison of human chorionic gonadotropin measurements by radioimmunoassay and in vitro bioassay - PubMed
We will start performing this method on our production HCG batches in around 4 weeks, when we can recheck and compare our results with your HPLC results and can discuss further. But as I stated in my previous email, most of the producers/sellers I know, have problem with your HCG results lately showing much less than it really is inside and this affects sales because end users believe in your test results. This is mainstream issue, not coming only from my HCG.
Regards.
Jano:
Well, you used their work as an argument why I am wrong, so you sort of made it my business, so I did indeed feel the necessity to address that.
Radioimmunoassays are not really used anymore and the article you have linked in from 1985.
In fact, US pharmacopoeia from 2005 - thus 30 years newer - suggests, or suggested assaying HCG via injecting it into female rats and then measuring the mass of their uteri - USP Monographs: Chorionic Gonadotropin
I believe I have explained well enough why different methods can yield different results and I am in fact very honored to be trusted by the community.
I believe this discussion can help explain to the community why the results indeed are different. Would you, perhaps, give me a permission to share the educative parts of our discussion that are anonymized with the community?
Thank you.
Man:
Hello,
Regarding the HCG biological activity measurement using HPLC:
The point is not whether the method which we sent to you is old, the point is what method is used to assess the biological activity of HCG, be it: using rats uterus or using Leyding cells and expression of testosterone or cAMP, these are all valid methods of assessing bioactivity, with available publications, usage in pharmacopeia etc., HPLC is not one of these methods and there is very good reason why it is not used. If you think otherwise, that’s fine, as long as you can supply your used methods of testing which have publications and are widely accepted, and we can compare the results and have a starting point from where to discuss further.
Regarding sharing our conversation to the community, of course, but only after we finish it, we are still in the middle of it.
Kind regards.
Jano:
Hello,
saying that HPLC isn't a valid method, but having posted an HPLC analysis of HCG to support your argument just a few emails ago is not really something that looks like a good faith argument here.
Regarding RPHPLC there are multiple publications that I was able to check, such as: Analysis of the human chorionic gonadotropin protein at the intact level by HILIC-MS and comparison with RPLC-MS - PubMed
Hope this helps.
Man:
Hello,
Regarding the publication you sent:
I am not sure if you have read the publication which you sent, there is not any mention about bioactivity measurement using HPLC, if you would read that publication you’ll see why exactly no-one is using HPLC for that purpose, how many isoforms there is, how hard just to fingerprint them etc.
And regarding HPLC results which I sent from another independent lab from same batch of HCG vials, yes their method is also not approved anywhere, I simply sent it to show results difference.
Jano:
Hello,
well, I believe that publication underlined rather well both the fact that RP-HPLC analysis is quite well possible, although, I agree with you, complicated, and that the Analyza Bialek's report is not worth the paper it is printed on if they weren't able to separate even the hCG alpha and beta subunits.
Also, as it is mentioned, the recombinant hCG glycoforms are by far not as numerous as the hCG au naturel and we don't really need to fingerprint them, as we are not really interested in the glycation details.
Although, it is entirely possible, that given we use rhCG standard, this might introduce higher error with urine derived hCG, that I admit.
No one is using HPLC methods in Pharmacopoeias because Pharmacopoeias are legacy products and they are updated with a high regard to sort of 'backwards' compatibility.
I can assure you that if the Pharmacopoeias were not updated with a high regard to 'backwards' compatibility you wouldn't be seeing tests on living animals
However, I believe it might be appropriate to add an commentary to our reports, that our reports are comparable only to our reports and that other testing methods can and will yield different results.
Man:
Hello,
Yes HPLC analysis of HCG is possible, no doubt about that, but not in order to determine HCG activity.
Regarding the rHCG vs HCG, yes you are right in that regard that it is possible to better approximate bioactivity of rHCG using HPLC than human derived HCG, but then is the question, how do you know what HCG you receive for analysis, because the biggest share in the market which you are testing is human derived , the rest share of the market is rHCG and not all from the same host, there is CHO, yeast rHCG, so in order to approximate bioactivity of rHCG you have to have that host's rHCG as your standard with known bioactivity, which anyway has to be determined using other methods.
Regarding why no-one us using HPLC method in pharmacopeia you are not correct, as HPLC would be much cheaper and easier method to determine HCG bioactivity if it was possible, everyone would use it, if it would be possible to validate it for determination of HCG bioactivity in production or testing purposes, but no-one uses it because it is not suitable method for this particular protein, due to huge variation in glycolisation pattern and that pattern has big influence to bioactivity, so one protein with the same amount of mg/ml with one glycolisation pattern can have very different bioactivity with the same amount mg/ml with different glycolisation pattern.
I would not agree that no one is using hplc in pharmacopoeia, for lot's of proteins HPLC validation is the best and most important step in determination of protein characteristics, but not all proteins are the same. And in this regard you can not measure everything using the same scheme.
Regarding testing not only protein's but other medicine in animals and humans, that is done not because it's some legacy or backward way of doing things, but there is very good reason why it is done this way, (i think the animals in our days except small rodents etc., is not used in modern world for trials), because in order to see the full spectrum of the molecules influence on the target body(system), you have to use as similar as possible body(system) for the target body(system) intended, as there is so much pathways of interaction in the body(system) that the cheapest way to repeat what is happening in the body(system) intended is to use as similar as possible body(system), for human's it's primates but nowadays it is rarely used and after initial testing on small rodents testing continues on human's, with human consent and payment accordingly.
About adding your suggested commentary on HCG test repots, yes we agree and that would make the confusion more clear which is going around right now regarding your HCG testing results.
Regards.
Jano:
>Yes HPLC analysis of HCG is possible, no doubt about that, but not in order to determine HCG activity.
Yes, for activity that's merely an estimation. But would my clients understand if I listed the amount in micrograms? No.
So I have to keep the reports simple.
>Regarding the rHCG vs HCG, yes you are right in that regard that it is possible to better approximate bioactivity of rHCG using HPLC than human derived HCG, but then is the question, how do you know what HCG you receive for analysis, because the biggest share in the market which you are testing is human derived , the rest share of the market is rHCG and not all from the same host, there is CHO, yeast rHCG, so in order to approximate bioactivity of rHCG you have to have that host's rHCG as your standard with known bioactivity, which anyway has to be determined using other methods.
For hdHCG the high amount of glycosilation patterns is observable. Regarding the rest, there's a tradeoff between keeping the testing accessible and keeping the testing perfect. I find that our margin of error is perfectly acceptable to all of our clients.
>Regarding why no-one us using HPLC method in pharmacopeia you are not correct, as HPLC would be much cheaper and easier method to determine HCG bioactivity if it was possible, everyone would use it, if it would be possible to validate it for determination of HCG bioactivity in production or testing purposes, but no-one uses it because it is not suitable method for this particular protein, due to huge variation in glycolisation pattern and that pattern has big influence to bioactivity, so one protein with the same amount of mg/ml with one glycolisation pattern can have very different bioactivity with the same amount mg/ml with different glycolisation pattern.
Correct. However I do believe that an accessible, 180 USD, however imperfect test has way more value to people than perfect 5000 USD test.
If you believe my clients are after the perfect 5000 USD test more than after an imperfect +-10/20% test for 180 USD for HCG, it is a free market and you can start the testing company focused on delivering the perfect HCG testing. I will keep on doing what I believe is the best for my clients.
>I would not agree that no one is using hplc in pharmacopoeia, for lot's of proteins HPLC validation is the best and most important step in determination of protein characteristics, but not all proteins are the same. And in this regard you can not measure everything using the same scheme.
You are right, I misconstructed the argument - I meant no one is using cutting edge HPLC methods, because of having to maintain legacy compatibility.
Naturally, I did not mean that HPLC methods are not used in Pharmacopoeias at all.
>Regarding testing not only protein's but other medicine in animals and humans, that is done not because it's some legacy or backward way of doing things, but there is very good reason why it is done this way, (i think the animals in our days except small rodents etc., is not used in modern world for trials), because in order to see the full spectrum of the molecules influence on the target body(system), you have to use as similar as possible body(system) for the target body(system) intended, as there is so much pathways of interaction in the body(system) that the cheapest way to repeat what is happening in the body(system) intended is to use as similar as possible body(system), for human's it's primates but nowadays it is rarely used and after initial testing on small rodents testing continues on human's, with human consent and payment accordingly.
However, that's more for preclinical testing or, in pharmacological settings, for introduction of manufacture, not routine analysis, which is what I'm usually doing.
Do you, actually, conduct the testing as per the pharmacopoeia?
Anyway, I will add the commentary to my HCG testing that Janoshik HCG tests are only comparable to other Janoshik HCG tests and may differ from bioactivity testing on rodents once I have the time.
Thank you for a fruitful discussion, but there is only so much time I can spend on a singular topic, so I believe we can assume our discussion to be concluded.
Have a good weekend.
Man:
Hello,
Thanks for the reply and stating all the facts.
Yes please let me know when you can add the commentary to HCG lab results, this is important for the clients and for the people who use their HCG. Can you do this week?
Also as we discussed previously, you can publish our conversation for public for them to be fully aware of HCG testing methods and accuracy of different testing methods, especially HPLC testing method for HCG.
Regards.
Jano:
Hello,
unfortunately, I cannot start with adding the commentary I mentioned to the reports this week, as the commentary is already being added to reports of HCG since about 10 days ago already
Thank you, will be doing so shortly. Hope you have a good day!
- Jano