Type-IIx
Member
A Profile of MENT (Trestolone)
Author: Type-IIx
Personally, I believe MENT is a particularly suppressive compound comparable to testosterone+etonogestrel in its HPG axis suppression. It may provoke a significant rise in systolic blood pressure versus replacement T dosages [1]. However, I know it is gaining popularity quickly despite a paucity of evidence on tolerability, recovery of HPG axis function post-cycle, so as to make it difficult to make appropriate risk-balancing considerations. My personal belief is that you may want to steer clear of MENT, at least until more data is available, if you intend to cycle off (i.e., you do not intend to BnC forever).
Putting my personal view aside, here's my findings on MENT with literature and the detailed anecdotes of a veteran that I respect a great deal for his experience with AAS generally, and MENT in particular:
Trestolone; Methylnortestosterone; 7 alpha-methyl-19-nortestosterone
Non-5α-reducible; particularly estrogenic (aromatizes to 7α-ME), progestid
Ref. p. 347 (330) of Llewellyn
[Conservative] Dose: Under 10 mg daily - with an acetate ester, typically 10-20 mg e2-3d
Regarding potency, consider that cheque drops (mibolerone) is the 17α-methylated form of MENT.
7α-methylation
7α-methylation serves to increase the anabolic potency of MENT by:
- ↑AR affinity [2]
- ↓reduction of the delta-4 (Δ⁴) bond ∴ delta-5(10) isomers are the major excreted metabolites (hindering metabolic inactivation) [3]
- ↓affinity for SHBG binding [4]
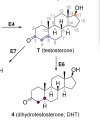
Aromatization

MENT's aromatic product (7α-methylestradiol; 7α-ME) is more than 4x(!) as potent ("efficacious," a bad thing here) in ER-containing cells [5]. Efficacy is determined by measuring the effect, e.g., growth (here, in breast cancer cells). The EC₅₀ (EC50) is determined by the concentrations at which the ligand triggers growth (this may be confirmed by measurements of cell cycle progression (i.e., the S-phase entry during the cell cycle).
The binding affinity (IC₅₀) of MENT's aromatic product (7α-methylestradiol) is 102% that of estradiol [5] which is typically used as the reference compound for ER binding given its noteworthy efficacy, potency, and affinity for the ERα receptor, in the literature.
Given the findings of Attardi et al, in comparing the rate of aromatization between MENT and Nandrolone, that "At 180 min, about 23% of MENT was converted to 7α-ME and about 13% of 19-NT to E2," knowing that Nandrolone aromatizes at 20% the rate of T [6], we can deduce that MENT aromatizes at roughly 35% the rate of T... to 7α-ME (an aromatic product with four-fold E2's potency, i.e., for causing growth in breast cancer cells). Simple multiplication of the rate of aromatization (35%) * EC50(7α-methylestradiol) * RBA(7α-methylestradiol) ≈ a 40% greater growth potential in ER-containing cells than T.
This would seem to support the anecdotes that MENT is quite a potent gynecomastic agent. With consideration of practical use, if the MENT dosage is ~70% the Test dosage it's on par with the estrogenicity of T, and given the aromatization to 7α-ME rather than E2, this consequence is unlikely to be reflected in bloodwork results. By way of comparison, e.g.: 35mg daily of MENT E ≈ as estrogenic as 350mg of Test E weekly (sans data on metabolism).
And MENT does also has progestational activity to take into consideration.
SHBG
Despite MENT's lack of affinity for SHBG:
The dose-response relationship was not established between MENT and SHBG.
Another study [8] confirmed the SHBG reduction from MENT:
[4] shows MENT a weak SHBG ligand (virtually no binding to SHBG after acute administration)
Joints
Nothing quite provides the joint relief of nandrolone, however said joint relief can be achieved with just 150-200mgs ran along side any cycle. NPP being preferable for more mg per mg of actual compound but still. Do I note a subtle different in my knees when running just trestolone versus my baseline? Yeah but one tenth that of incorporating 200mgs of deca/npp.
However in the past running 100mg every day of ment ace, was comparable to 200-250mg of npp per day regarding performance metrics and lean tissue growth. Actually probably more likened to 150-200mg NPP ED run alongside Anadrol. You lack the aggression of tren, so you don’t have that “not heavy enough” attitude, but the weights you were moving will move a lot faster, and for more reps. You’re not going to tire out very quickly at all.
Ideally for your first ment cycle if you have joint issues, so long as you don’t have issues running 19nors, I would run 4 weeks at 25mg/day or 50mg/eod of ment, and at 3-4 weeks once you’ve seen how you’re going to react to ment add in 25mg/day or 50mg EOD of npp. Understand that both of these compound will compete at the receptor and testosterone will just be left to convert to estradiol or DHT. When running something like trestolone I find it but to run 100-150mg (TRT dose) of test along side but not more. If you have issues with 19 Nors, this drug will not be pleasant. This is the only drug that at high doses made me lactate and require prami.
Dosage
25mg per day or 50mg EOD to start. Twice weekly even for Trest E is too infrequent.
This dosage speculation [from Llewellyn] is largely based on the fact that a therapeutic replacement dose was less than 1mg per day, however in practice it really doesn't translate well. No, 1mg per day of testolone acetate is not going to replace a 100-200mg TRT dosage in the grand scheme of things. One of the primary factors in it being dropped was that at such a dosage there was insufficient estradiol conversion and activity at such dosages. While it is very anabolic, in terms of taking to improve performance metrics a higher dose is needed. Goals as well as experience and tolerability of a compound for you the individual come into play. Also keep in mind that just because at X dose its 10 fold stronger than testosterone, doesn't mean that at higher dosages that scales evenly. I would say 3:1 to 5:1 depending on how you respond once your in the 200-400mg range, not 10:1. The beauty of ment for me would be the mineral retention, and insanely rapid glycogen replenishment (diet supporting of course). I will tell you that running 700mg/week in the past that it was not equal to running 7 grams of test (10:1), nor 3500mg of test (5:1), but more like 2.5-3 grams at most.
Side-Effects
blood pressure
Ment is very powerful, and can have insane sides if you don't keep your levels relatively stable. Also your diet is of the utmost importance.
Important considerations: OFFSEASON/OFFCYCLE blood pressure & resting heart rate
Treat this like doing your first tren cycle. Once you find out how you tolerate the drug you can experiment with your dosing frequency, but not before then.
And no I am not trying to scare anyone. It can be an extremely well tolerated and effective drug if run properly. As with everything though, this comes down to the user and all outside factors. The harsher the compound, the more this comes into effect.
core temperature
Any form of trestolone will increase your body temp. You will note this within 1-3 weeks of starting. Night sweats are to be expected.
libido, erectile function
Very high but not like the tren androgen "rapey" mood. Far more pleasant and controllable. I haven't had ED while on any 19nor so I can't speak on that. That being said, just like any 19nor if your prolactin levels get too high you're going to have issues coming to climax. Keeping estrogen at your happy level whatever that is, and your prolactin in check will alleviate this.
appetite
definitely increased but again I wouldn't say I'm going hypoglycemic like I do on tren. Just wanting more food in general.
liver
While I will need to censor some things in my lab work to post if I decide, I did find some of my older labs from a ment cycle to answer this question. My baseline AST/ALTs are in the low to mid teens generally. On cycle, they rose to 32 and 38 respectively.
blood pressure
This is a big one. My offseason/offcycle BP is generally 110 over 60 or lower. This morning it was 118 over 76. Previous experiences for me with ment I will say it would get higher than I like but not dangerously so. Mind you I do have a lot of ancillary supplements that I take religiously, but on ment my resting BP with never goes over 130/90, ever. That said, there is a noted increase that people definitely need to pay attention to. If you drink, if you eat like shit, or just are genetically predisposed, this drug will seriously mess with your BP. No different than a high dose anadrol cycle.
pumps
I hate them, some love them. You're going to have very full muscles and naturally very strong pumps. This is a pro for some, and really the only major con of AAS for me. This mornings run sucked, I will be wearing compression wear to minimize calf pumps from now on.
digestive (nausea/acid reflux)
While tren and nandrolone give me acid reflux to some extent, I honestly have never had severe issues there while on trestolone. Everyone is different here but I know a few people that can not run nandrolone or tren due to that issue.
PR binding
Regarding its progestational activity, its actually not that different than its ability to bind to the androgen receptor. Is is a very strong progestin. Much more so than tren, or nandrolone. More comparable to medroxyprogesterone acetate. Given its affinity to the receptor site, mifepristone really isnt strong enough to inhibit the effects completely. Noting this, yes it is absolutely essential to have either caber or prami on hand if you're sensitive to progesterone related side effects, well more specifically prolactin.
Remember this was in research as a form of MALE BIRTH CONTROL. Its harsh progestational nature was and is a priority to have this effect. This is why I liken its activity as similar to medroxyprogesterone which is 10-15x the progestin in comparison to progesterone. An anti-progestin that is designed to knock off natural progesterone isn't going to be able to compete with a lot of the modern synthetics. Much in the sense that you could take halotestin and bicalutamide (anti androgen) together and still have androgenic rage...
_______________________________________
References:
[1] Walton, M. J., Kumar, N., Baird, D. T., Ludlow, H., & Anderson, R. A. (2007). 7 -Methyl-19-Nortestosterone (MENT) vs Testosterone in Combination With Etonogestrel Implants for Spermatogenic Suppression in Healthy Men. Journal of Andrology, 28(5), 679–688. doi:10.2164/jandrol.107.002683
[2] Fragkaki, A. G., Angelis, Y. S., Koupparis, M., Tsantili-Kakoulidou, A., Kokotos, G., & Georgakopoulos, C. (2009). Structural characteristics of anabolic androgenic steroids contributing to binding to the androgen receptor and to their anabolic and androgenic activities. Steroids, 74(2), 172–197. doi:10.1016/j.steroids.2008.10.016
[3] Recent advances in doping analysis (12)... Koln (2004): 261 - 268
[4] Kumar, N., Suvisaari, J., Tsong, Y., Aguillaume, C., Wayne, C. et al. (1997). Pharmacokinetics of 7alpha-methyl-19-nortestosterone in men and cynmolgus monkeys. Journal of Andrology, 18(4), 352-358.
[5] Attardi BJ, Pham TC, Radler LC, Burgenson J, Hild SA, Reel JR. Dimethandrolone (7alpha,11beta-dimethyl-19-nortestosterone) and 11beta-methyl-19-nortestosterone are not converted to aromatic A-ring products in the presence of recombinant human aromatase. J Steroid Biochem Mol Biol. 2008;110(3-5):214-222. doi:10.1016/j.jsbmb.2007.11.009
[6] Ryan, Kenneth J. “Biological aromatization of steroids.” Journal of Biological Chemistry 234.2 (1959): 268-272.
[7] Suvisaari, J., Sundaram, K., Noe, G., Kumar, N., Aguillaume, C., Tsong, Y. Y., … Bardin, C. W. (1997). Pharmacokinetics and pharmacodynamics of 7alpha-methyl-19- nortestosterone after intramuscular administration in healthy men. Human Reproduction, 12(5), 967–973. doi:10.1093/humrep/12.5.967
[8] Noé, G., Suvisaari, J., Martin, C., Moo-Young, A. J., Sundaram, K., Saleh, S. I., … Lähteenmäki, P. (1999). Gonadotrophin and testosterone suppression by 7α-methyl-19-nortestosterone acetate administered by subdermal implant to healthy men. Human Reproduction, 14(9), 2200–2206. doi:10.1093/humrep/14.9.2200
Author: Type-IIx
Personally, I believe MENT is a particularly suppressive compound comparable to testosterone+etonogestrel in its HPG axis suppression. It may provoke a significant rise in systolic blood pressure versus replacement T dosages [1]. However, I know it is gaining popularity quickly despite a paucity of evidence on tolerability, recovery of HPG axis function post-cycle, so as to make it difficult to make appropriate risk-balancing considerations. My personal belief is that you may want to steer clear of MENT, at least until more data is available, if you intend to cycle off (i.e., you do not intend to BnC forever).
Putting my personal view aside, here's my findings on MENT with literature and the detailed anecdotes of a veteran that I respect a great deal for his experience with AAS generally, and MENT in particular:
Trestolone; Methylnortestosterone; 7 alpha-methyl-19-nortestosterone
Non-5α-reducible; particularly estrogenic (aromatizes to 7α-ME), progestid
Ref. p. 347 (330) of Llewellyn
[Conservative] Dose: Under 10 mg daily - with an acetate ester, typically 10-20 mg e2-3d
Regarding potency, consider that cheque drops (mibolerone) is the 17α-methylated form of MENT.
7α-methylation
7α-methylation serves to increase the anabolic potency of MENT by:
- ↑AR affinity [2]
- ↓reduction of the delta-4 (Δ⁴) bond ∴ delta-5(10) isomers are the major excreted metabolites (hindering metabolic inactivation) [3]
- ↓affinity for SHBG binding [4]
Aromatization

MENT's aromatic product (7α-methylestradiol; 7α-ME) is more than 4x(!) as potent ("efficacious," a bad thing here) in ER-containing cells [5]. Efficacy is determined by measuring the effect, e.g., growth (here, in breast cancer cells). The EC₅₀ (EC50) is determined by the concentrations at which the ligand triggers growth (this may be confirmed by measurements of cell cycle progression (i.e., the S-phase entry during the cell cycle).
The binding affinity (IC₅₀) of MENT's aromatic product (7α-methylestradiol) is 102% that of estradiol [5] which is typically used as the reference compound for ER binding given its noteworthy efficacy, potency, and affinity for the ERα receptor, in the literature.
Given the findings of Attardi et al, in comparing the rate of aromatization between MENT and Nandrolone, that "At 180 min, about 23% of MENT was converted to 7α-ME and about 13% of 19-NT to E2," knowing that Nandrolone aromatizes at 20% the rate of T [6], we can deduce that MENT aromatizes at roughly 35% the rate of T... to 7α-ME (an aromatic product with four-fold E2's potency, i.e., for causing growth in breast cancer cells). Simple multiplication of the rate of aromatization (35%) * EC50(7α-methylestradiol) * RBA(7α-methylestradiol) ≈ a 40% greater growth potential in ER-containing cells than T.
Keep in mind that you also need to consider the rate of breakdown of the produced 7a-methylestradiol, as well as the estrogenic potency of the resulting metabolites (Peter Bond, Oct 20 2021)
This would seem to support the anecdotes that MENT is quite a potent gynecomastic agent. With consideration of practical use, if the MENT dosage is ~70% the Test dosage it's on par with the estrogenicity of T, and given the aromatization to 7α-ME rather than E2, this consequence is unlikely to be reflected in bloodwork results. By way of comparison, e.g.: 35mg daily of MENT E ≈ as estrogenic as 350mg of Test E weekly (sans data on metabolism).
And MENT does also has progestational activity to take into consideration.
SHBG
Despite MENT's lack of affinity for SHBG:
[7]...clear decreases in SHBG concentrations...mean serum SHBG concentration of all 24 subjects decreased from 31 ± 2.2 (baseline) to 26 ± 2.1 nmol/l (two days after the last injection). This decrease in SHBG concentrations after the i.m. injections was significant...
The dose-response relationship was not established between MENT and SHBG.
Another study [8] confirmed the SHBG reduction from MENT:
SHBG concentrations decreased significantly in the groups that used two and four implants (P=0.043 and 0.001, respectively) (Figure 2)
[4] shows MENT a weak SHBG ligand (virtually no binding to SHBG after acute administration)
Joints
Nothing quite provides the joint relief of nandrolone, however said joint relief can be achieved with just 150-200mgs ran along side any cycle. NPP being preferable for more mg per mg of actual compound but still. Do I note a subtle different in my knees when running just trestolone versus my baseline? Yeah but one tenth that of incorporating 200mgs of deca/npp.
However in the past running 100mg every day of ment ace, was comparable to 200-250mg of npp per day regarding performance metrics and lean tissue growth. Actually probably more likened to 150-200mg NPP ED run alongside Anadrol. You lack the aggression of tren, so you don’t have that “not heavy enough” attitude, but the weights you were moving will move a lot faster, and for more reps. You’re not going to tire out very quickly at all.
Ideally for your first ment cycle if you have joint issues, so long as you don’t have issues running 19nors, I would run 4 weeks at 25mg/day or 50mg/eod of ment, and at 3-4 weeks once you’ve seen how you’re going to react to ment add in 25mg/day or 50mg EOD of npp. Understand that both of these compound will compete at the receptor and testosterone will just be left to convert to estradiol or DHT. When running something like trestolone I find it but to run 100-150mg (TRT dose) of test along side but not more. If you have issues with 19 Nors, this drug will not be pleasant. This is the only drug that at high doses made me lactate and require prami.
Dosage
25mg per day or 50mg EOD to start. Twice weekly even for Trest E is too infrequent.
This dosage speculation [from Llewellyn] is largely based on the fact that a therapeutic replacement dose was less than 1mg per day, however in practice it really doesn't translate well. No, 1mg per day of testolone acetate is not going to replace a 100-200mg TRT dosage in the grand scheme of things. One of the primary factors in it being dropped was that at such a dosage there was insufficient estradiol conversion and activity at such dosages. While it is very anabolic, in terms of taking to improve performance metrics a higher dose is needed. Goals as well as experience and tolerability of a compound for you the individual come into play. Also keep in mind that just because at X dose its 10 fold stronger than testosterone, doesn't mean that at higher dosages that scales evenly. I would say 3:1 to 5:1 depending on how you respond once your in the 200-400mg range, not 10:1. The beauty of ment for me would be the mineral retention, and insanely rapid glycogen replenishment (diet supporting of course). I will tell you that running 700mg/week in the past that it was not equal to running 7 grams of test (10:1), nor 3500mg of test (5:1), but more like 2.5-3 grams at most.
Side-Effects
blood pressure
Ment is very powerful, and can have insane sides if you don't keep your levels relatively stable. Also your diet is of the utmost importance.
Important considerations: OFFSEASON/OFFCYCLE blood pressure & resting heart rate
Treat this like doing your first tren cycle. Once you find out how you tolerate the drug you can experiment with your dosing frequency, but not before then.
And no I am not trying to scare anyone. It can be an extremely well tolerated and effective drug if run properly. As with everything though, this comes down to the user and all outside factors. The harsher the compound, the more this comes into effect.
core temperature
Any form of trestolone will increase your body temp. You will note this within 1-3 weeks of starting. Night sweats are to be expected.
libido, erectile function
Very high but not like the tren androgen "rapey" mood. Far more pleasant and controllable. I haven't had ED while on any 19nor so I can't speak on that. That being said, just like any 19nor if your prolactin levels get too high you're going to have issues coming to climax. Keeping estrogen at your happy level whatever that is, and your prolactin in check will alleviate this.
appetite
definitely increased but again I wouldn't say I'm going hypoglycemic like I do on tren. Just wanting more food in general.
liver
While I will need to censor some things in my lab work to post if I decide, I did find some of my older labs from a ment cycle to answer this question. My baseline AST/ALTs are in the low to mid teens generally. On cycle, they rose to 32 and 38 respectively.
blood pressure
This is a big one. My offseason/offcycle BP is generally 110 over 60 or lower. This morning it was 118 over 76. Previous experiences for me with ment I will say it would get higher than I like but not dangerously so. Mind you I do have a lot of ancillary supplements that I take religiously, but on ment my resting BP with never goes over 130/90, ever. That said, there is a noted increase that people definitely need to pay attention to. If you drink, if you eat like shit, or just are genetically predisposed, this drug will seriously mess with your BP. No different than a high dose anadrol cycle.
pumps
I hate them, some love them. You're going to have very full muscles and naturally very strong pumps. This is a pro for some, and really the only major con of AAS for me. This mornings run sucked, I will be wearing compression wear to minimize calf pumps from now on.
digestive (nausea/acid reflux)
While tren and nandrolone give me acid reflux to some extent, I honestly have never had severe issues there while on trestolone. Everyone is different here but I know a few people that can not run nandrolone or tren due to that issue.
PR binding
Regarding its progestational activity, its actually not that different than its ability to bind to the androgen receptor. Is is a very strong progestin. Much more so than tren, or nandrolone. More comparable to medroxyprogesterone acetate. Given its affinity to the receptor site, mifepristone really isnt strong enough to inhibit the effects completely. Noting this, yes it is absolutely essential to have either caber or prami on hand if you're sensitive to progesterone related side effects, well more specifically prolactin.
Remember this was in research as a form of MALE BIRTH CONTROL. Its harsh progestational nature was and is a priority to have this effect. This is why I liken its activity as similar to medroxyprogesterone which is 10-15x the progestin in comparison to progesterone. An anti-progestin that is designed to knock off natural progesterone isn't going to be able to compete with a lot of the modern synthetics. Much in the sense that you could take halotestin and bicalutamide (anti androgen) together and still have androgenic rage...
_______________________________________
References:
[1] Walton, M. J., Kumar, N., Baird, D. T., Ludlow, H., & Anderson, R. A. (2007). 7 -Methyl-19-Nortestosterone (MENT) vs Testosterone in Combination With Etonogestrel Implants for Spermatogenic Suppression in Healthy Men. Journal of Andrology, 28(5), 679–688. doi:10.2164/jandrol.107.002683
[2] Fragkaki, A. G., Angelis, Y. S., Koupparis, M., Tsantili-Kakoulidou, A., Kokotos, G., & Georgakopoulos, C. (2009). Structural characteristics of anabolic androgenic steroids contributing to binding to the androgen receptor and to their anabolic and androgenic activities. Steroids, 74(2), 172–197. doi:10.1016/j.steroids.2008.10.016
[3] Recent advances in doping analysis (12)... Koln (2004): 261 - 268
[4] Kumar, N., Suvisaari, J., Tsong, Y., Aguillaume, C., Wayne, C. et al. (1997). Pharmacokinetics of 7alpha-methyl-19-nortestosterone in men and cynmolgus monkeys. Journal of Andrology, 18(4), 352-358.
[5] Attardi BJ, Pham TC, Radler LC, Burgenson J, Hild SA, Reel JR. Dimethandrolone (7alpha,11beta-dimethyl-19-nortestosterone) and 11beta-methyl-19-nortestosterone are not converted to aromatic A-ring products in the presence of recombinant human aromatase. J Steroid Biochem Mol Biol. 2008;110(3-5):214-222. doi:10.1016/j.jsbmb.2007.11.009
[6] Ryan, Kenneth J. “Biological aromatization of steroids.” Journal of Biological Chemistry 234.2 (1959): 268-272.
[7] Suvisaari, J., Sundaram, K., Noe, G., Kumar, N., Aguillaume, C., Tsong, Y. Y., … Bardin, C. W. (1997). Pharmacokinetics and pharmacodynamics of 7alpha-methyl-19- nortestosterone after intramuscular administration in healthy men. Human Reproduction, 12(5), 967–973. doi:10.1093/humrep/12.5.967
[8] Noé, G., Suvisaari, J., Martin, C., Moo-Young, A. J., Sundaram, K., Saleh, S. I., … Lähteenmäki, P. (1999). Gonadotrophin and testosterone suppression by 7α-methyl-19-nortestosterone acetate administered by subdermal implant to healthy men. Human Reproduction, 14(9), 2200–2206. doi:10.1093/humrep/14.9.2200