Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
Style variation
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
[R.C. Labs ] [Tren e] - [Mass Spec] - [2014-09] [Angus]
- Thread starter Capt Forest
- Start date
So I hooked up with a guy named Jonathan Hilmer and had him check out the reports that angus did for me Here is his analysis. His credentials are listed at the bottom of the page. This
Jonathan Hilmer:
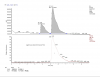
"The analytical report you attached is a really basic HPLC analysis with UV
and MS. The UV is show in the "top" (left) of the first page. Any
steroid-like molecule should have some UV signal, so that's a nice general
measurement of similar compounds. The "bottom" (right) of the first page
shows a TIC (total ion current or total ion chromatogram): that signal is
coming from their MS, and it's collected simultaneously with the UV data.
The TIC is by definition the total of all the ions they are detecting via
MS. The second page shows the individual ions that were detected between 5
and 6 minutes (averaged over that time). It shows that most all of the
signal is coming from one ion, which is m/z=383.13. The labels on that
page also tell you that it is APCI (atmospheric pressure chemical
ionization) MS, which is a technique used to turn the molecules into ions:
molecules MUST be ionized to be detected! If you don't ionize a molecule,
you don't detect it. APCI is good for making ions from molecules which
don't ionize well, like steroids. Other techniques, like ESI, have a lower
ionization efficiency. No technique will make 100% of the molecules into
ions.
That report is a little messy. The UV peak at 3.82 shows that something
else is present, but it didn't show up on the MS trace (didn't ionize
well), so we don't know what it is. It's likely a barely-functionalized
aromatic compound (like naphthalene, but not that particular molecule)
because it has UV but no MS trace. The UV peak at 5.31 is probably coming
from the 383.13 molecule. Using the MS trace to calculate purity, you'd
probably get 98+%, but if you use the UV you might call it ~60% (I'm not
actually calculating the numbers here). Which is more relevant? In this
case, UV is better because it shows more, but neither is really very good.
If you were to take the same sample and add many grams of the right kind of
lipids, you wouldn't see any lipid on the UV (no UV absorbance for that
molecule) and you wouldn't see lipid on the MS because it wouldn't come off
the column. So your purity would look good via UV and MS (nice clean peaks
for the 383.13 molecule), and the total weight of the sample would be large
(due to the added lipid), but the real purity would be TERRIBLE: an easy
scam. Getting an accurate determination of purity of a sample which has
been intentionally manipulated like that would be really tough: as I said
above, it requires that you throw a lot of tests at it to be sure.
The best analysis for problems like yours would be NMR because it's
inherently quantitative and it's almost impossible to fail to detect
molecules, so contaminants can be detected. The problem is that it
requires fairly high concentrations of material."
Capt Forest:
"In your opinion you think that the reports that i have shown you are not accurate or a scam at best? I am very skeptical that one report came back at 100% purity i believe its the one you described in the previous messgae.
The other report came back at 74% purity, a much more reasonable number. This sample was half ml oil. The one that came back at 100% was a 10mg pill of oxandrolone. In your opinion how much merit should i put into each of these reports?"
Jonathan Hilmer:
"Capt Forest,
The reports are fine for what they are: a reasonable analysis but not the
full picture. You should assume that the number means "74%" of X,Y,Z or
considering complications A,B,C. In this particular case, it's 74% via UV
(which wavelength? I can't find it there, and it's important) for
molecules which could elute off the column in that time window. That's
actually fairly comprehensive, but as I said it still would be easy to
intentionally manipulate the same to produce that result. If you're
worried about that possibility, then this report isn't enough. The MS on
that report shows that the molecule in question weighs 383, so it is NOT
oxandrolone. The fact it has a big UV peak also confirms that.
The other report shows that oxandrolone is definitely present, but what are
the other peaks? They could be background ions, in which case they can be
ignored. Or they could be contaminants in your sample: oxidized
oxandrolone would show up as a new peak in the spectrum. They might be
+H2O, which is something that can happen with electrospray, and it doesn't
mean anything regarding your sample. That report also shows only a tiny UV
peak, which is what you'd expect, but there is definitely another peak
present at 3.79 min, so it can't really be 100% pure.
Jonathan
Jonathan Hilmer, Ph.D.
Facility Coordinator
Mass Spectrometry Facility
Department of Chemistry and Biochemistry
Montana State University
Bozeman, MT 59717"
I have no problem posting screen shots for people that might be skeptical of this conversation. This is also posted in the MS report on the legend anavar.
Jonathan Hilmer:
"The analytical report you attached is a really basic HPLC analysis with UV
and MS. The UV is show in the "top" (left) of the first page. Any
steroid-like molecule should have some UV signal, so that's a nice general
measurement of similar compounds. The "bottom" (right) of the first page
shows a TIC (total ion current or total ion chromatogram): that signal is
coming from their MS, and it's collected simultaneously with the UV data.
The TIC is by definition the total of all the ions they are detecting via
MS. The second page shows the individual ions that were detected between 5
and 6 minutes (averaged over that time). It shows that most all of the
signal is coming from one ion, which is m/z=383.13. The labels on that
page also tell you that it is APCI (atmospheric pressure chemical
ionization) MS, which is a technique used to turn the molecules into ions:
molecules MUST be ionized to be detected! If you don't ionize a molecule,
you don't detect it. APCI is good for making ions from molecules which
don't ionize well, like steroids. Other techniques, like ESI, have a lower
ionization efficiency. No technique will make 100% of the molecules into
ions.
That report is a little messy. The UV peak at 3.82 shows that something
else is present, but it didn't show up on the MS trace (didn't ionize
well), so we don't know what it is. It's likely a barely-functionalized
aromatic compound (like naphthalene, but not that particular molecule)
because it has UV but no MS trace. The UV peak at 5.31 is probably coming
from the 383.13 molecule. Using the MS trace to calculate purity, you'd
probably get 98+%, but if you use the UV you might call it ~60% (I'm not
actually calculating the numbers here). Which is more relevant? In this
case, UV is better because it shows more, but neither is really very good.
If you were to take the same sample and add many grams of the right kind of
lipids, you wouldn't see any lipid on the UV (no UV absorbance for that
molecule) and you wouldn't see lipid on the MS because it wouldn't come off
the column. So your purity would look good via UV and MS (nice clean peaks
for the 383.13 molecule), and the total weight of the sample would be large
(due to the added lipid), but the real purity would be TERRIBLE: an easy
scam. Getting an accurate determination of purity of a sample which has
been intentionally manipulated like that would be really tough: as I said
above, it requires that you throw a lot of tests at it to be sure.
The best analysis for problems like yours would be NMR because it's
inherently quantitative and it's almost impossible to fail to detect
molecules, so contaminants can be detected. The problem is that it
requires fairly high concentrations of material."
Capt Forest:
"In your opinion you think that the reports that i have shown you are not accurate or a scam at best? I am very skeptical that one report came back at 100% purity i believe its the one you described in the previous messgae.
The other report came back at 74% purity, a much more reasonable number. This sample was half ml oil. The one that came back at 100% was a 10mg pill of oxandrolone. In your opinion how much merit should i put into each of these reports?"
Jonathan Hilmer:
"Capt Forest,
The reports are fine for what they are: a reasonable analysis but not the
full picture. You should assume that the number means "74%" of X,Y,Z or
considering complications A,B,C. In this particular case, it's 74% via UV
(which wavelength? I can't find it there, and it's important) for
molecules which could elute off the column in that time window. That's
actually fairly comprehensive, but as I said it still would be easy to
intentionally manipulate the same to produce that result. If you're
worried about that possibility, then this report isn't enough. The MS on
that report shows that the molecule in question weighs 383, so it is NOT
oxandrolone. The fact it has a big UV peak also confirms that.
The other report shows that oxandrolone is definitely present, but what are
the other peaks? They could be background ions, in which case they can be
ignored. Or they could be contaminants in your sample: oxidized
oxandrolone would show up as a new peak in the spectrum. They might be
+H2O, which is something that can happen with electrospray, and it doesn't
mean anything regarding your sample. That report also shows only a tiny UV
peak, which is what you'd expect, but there is definitely another peak
present at 3.79 min, so it can't really be 100% pure.
Jonathan
Jonathan Hilmer, Ph.D.
Facility Coordinator
Mass Spectrometry Facility
Department of Chemistry and Biochemistry
Montana State University
Bozeman, MT 59717"
I have no problem posting screen shots for people that might be skeptical of this conversation. This is also posted in the MS report on the legend anavar.
mercury
New Member
I have pointed before that those reports are messy and not reliable, they are not done by credible lab but some student who has access to a lab and does not know too much about it.
Why don't more people use NMR?The best analysis for problems like yours would be NMR because it's
inherently quantitative and it's almost impossible to fail to detect
molecules, so contaminants can be detected. The problem is that it
requires fairly high concentrations of material.
It should be easy to have a lab to provide an NMR analysis of a sample without an interpretation. This is something @Bill Roberts suggested to me. The trick is to find qualified people to interpret it who are not associated with the lab.
@Capt Forest perhaps your guy could provide more info on this?
@Capt Forest perhaps your guy could provide more info on this?
Millard if you would like to write a question for me to ask i will be happy to play the medium.
BigAngus
New Member
I have pointed before that those reports are messy and not reliable, they are not done by credible lab but some student who has access to a lab and does not know too much about it.
The report that is posted in this thread is done by an independent, free standing lab in the US. They have no students in their facility whatsoever nor have they ever had any.
Similar threads
- Replies
- 1
- Views
- 316
- Replies
- 9
- Views
- 401