Testosterone for Women: Waiting for the Female Aphrodisiac
Testosterone for Women: Waiting for the Female Aphrodisiac by John Hoberman, PhD
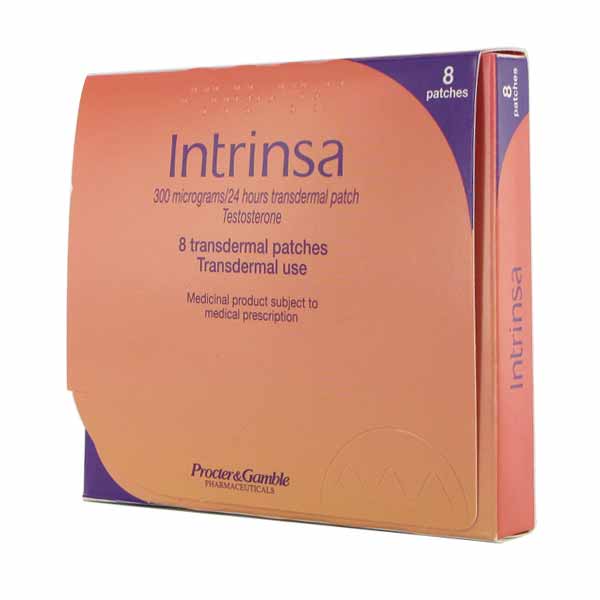
In December 2004 a U.S. Food and Drug Administration (FDA) Advisory Committee for Reproductive Health Drugs refused to recommend Procter & Gamble's Intrinsa testosterone patch for female sexual dysfunction. The FDA panel found that administering testosterone to these patients would produce an average of one extra sexual encounter a month, a benefit that in the panel's view did not justify exposing women to the risk of heart attacks and strokes. This decision surprised executives at both Procter & Gamble and at BioSante Pharmaceuticals, Inc., its principal rival in the race to develop a mass-marketed male hormone sex stimulant for women. BioSante had been rooting for P& G's application because it believed its own testosterone delivery systems gels, creams, nasal sprays, and pills would outperform the Intrinsa transdermal testosterone patch both as drug delivery vehicles and thus in the marketplace.
 thinksteroids.com
thinksteroids.com
Testosterone for Women: Waiting for the Female Aphrodisiac by John Hoberman, PhD
In December 2004 a U.S. Food and Drug Administration (FDA) Advisory Committee for Reproductive Health Drugs refused to recommend Procter & Gamble's Intrinsa testosterone patch for female sexual dysfunction. The FDA panel found that administering testosterone to these patients would produce an average of one extra sexual encounter a month, a benefit that in the panel's view did not justify exposing women to the risk of heart attacks and strokes. This decision surprised executives at both Procter & Gamble and at BioSante Pharmaceuticals, Inc., its principal rival in the race to develop a mass-marketed male hormone sex stimulant for women. BioSante had been rooting for P& G's application because it believed its own testosterone delivery systems gels, creams, nasal sprays, and pills would outperform the Intrinsa transdermal testosterone patch both as drug delivery vehicles and thus in the marketplace.

Testosterone for Women: Waiting for the Female Aphrodisiac
In December 2004 a U.S. Food and Drug Administration (FDA) Advisory Committee for Reproductive Health Drugs refused to recommend Procter & Gamble's
 thinksteroids.com
thinksteroids.com

