Best homebrew step by step ever! No need to overthink things.Don't bother.
Weigh the compound.
Measure the BA/BB with syringes.
Throw it all in the beaker.
Heat to 50c max. Swirl.
Optionally, get a magnetic stirrer with hotplate.
View attachment 142963
Done.
Nothing will happen to any of the solvents or to the oil lmao.
Check solvents' boiling points and see if you are even getting close at 50c.
Check compound's melting point and see if you are even getting close at 50c.
How:
You want information about trenbolone acetate.
You google Trenbolone acetate CAS Number.
(CAS number = Chemical Abstracts Service number.)
10161-34-9 is the CASRN of trenbolone acetate.
You google 10161-34-9 melting point.
You check multiple sources. No mistakes.
Keep the temperature way lower than that.
Half of that if you want.
50c suits all of the compounds I've done till now.
Tren A/E/H
Test C/P/E/U
Nandrolone D/NPP
Mast E/P
EQ
Primo E
1)20%BB
2)2%BA
*1 and 2 measured with a syringe and thrown right in. Straight away with no delays and shit,throw the carrier in. Get a graduated beaker and straight up fill it to the desired level with your carrier oil. No fancy numbers. Displacements my ass.
View attachment 142964
3)EO(What i use. Pick any) MCT,GSO,CSO
Stove will be hard to regulate the heat.
You will need a glass thermometer in the beaker all the time plus your eyes on it.
Invest in a magnetic stirrer hotplate.
It will cost you 60$.
You will buy one sooner or later. Make it sooner.
Ebay.
Syringe filters and bottle top filters. Alibaba.
1)PVDF 0.22um 4$ each bottle top filter. Case of 24. 3 years shelf life.
They sell the same ones on ebay. Literally the same fucking brand, 5+ times the alibaba price.
2)Syringe filters PVDF 0.22um 0.50$ each. Case of 50/100. 3 years shelf life.
Fuck 2.
Go straight to 1. You will sooner or later so let's make it sooner.
Gl45 Lower units to screw the filters on. You buy these separately. I hear the plastic ones crack. Ebay.
25$ vacuum pump from ebay.
View attachment 142962
Run the tubing to the filter. Use tensioner on the pump part. Make sure that there are no air leaks.
The pump will need a 12v converter. Costs pennies. Just get one and run the wiring.
Long esters 200mg/ml
Short esters 100mg/ml
Equipoise 500mg/ml minimum. Yeah I know,weird. No you won't have pip.
Just get over with it and brew it like a man at 650mg/ml. 0 pip. Equipoise raws are liquid in room temperature.
These concentrations are problems free and cause no issues. The solutions will be stable as fuck and pip-less.
Find the carrier oil that suits you.
If you are in the US and doing small batches,you can filter into ready presterilized vials. If you trust that they are correctly sterilized.
If you are in Europe,buying ready presterilized vials is expensive, and troublesome. Buy from eBay UK if you insist.
Otherwise, crimp your own.
I can help with this except from the sterilization.
There are many protocols to sterilize the vials,rubbers,aluminum seals. This is the only thing I will leave on you.
Yeah. Crimp your own. Fuck the precrimped ones.
I use an autoclave to sterilize the vials.
Ask anything you want.
View attachment 142965
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
Style variation
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Brewing question
- Thread starter Fergy
- Start date
Yep - you aren’t gonna get close to boiling BA or BB you will likely smoke your carrier oil or substance first.Ethyl Alcohol is not the same as Benzyl Alcohol.
Ethyl alcohol is Ethanol.
Benzyl Alcohol, CAS 100-51-6
View attachment 142969
Ethyl Alcohol-Ethanol, CAS 64-17-5
View attachment 142970
All of this is from the MSDS.
The rest is broscience.
FR0Z3N_B0MB3RRR
New Member
Yes and i mean.. you see 78 C as boiling point of Ethanolsius... but I see it evaporating at 25 C in my room.. so even if Boiling Point of BA is higher than room temp, I think it could easily evaporate Like ethanol... even if we have those data’s.. maybe “evaporatingness” isn’t related to boiling point... it’s rest day and I feel so tired for my 10 weekly training session... so don’t wanna search on web about boiling point and evaporatingness and neither the right term of evaporatingness that I think it has been invented by me now...Ethyl Alcohol is not the same as Benzyl Alcohol.
Ethyl alcohol is Ethanol.
Benzyl Alcohol, CAS 100-51-6
View attachment 142969
Ethyl Alcohol-Ethanol, CAS 64-17-5
View attachment 142970
All of this is from the MSDS.
The rest is broscience.
Put some water and ethanol into some surface and will see they ll disappear.. even if water boils at 100 C ... so, I don’t trust boiling points and will stick to cover my becker and put BA before filtering.. you can’t change my beliefs and setup Trenned.. ahaha I m thinking about your rant on “I know everything stuff” and laughing... I m not saying I know everything , but we re complicating this stuff a lot more than needed... ahahah... @fergie fuck off, it’s your fault... watch the madness you created with your fuckin brewing questions...
FR0Z3N_B0MB3RRR
New Member
Jokes aside, couldn’t resist and searched
Q: What’s the difference between evaporation and boiling? | NSTA
very interesting lecture...


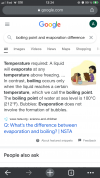




Q: What’s the difference between evaporation and boiling? | NSTA
very interesting lecture...








FR0Z3N_B0MB3RRR
New Member
@TrennedOutLunatic and remember, Florentina is ready for work...Jokes aside, couldn’t resist and searched
Q: What’s the difference between evaporation and boiling? | NSTA
very interesting lecture...
View attachment 142971View attachment 142972View attachment 142973View attachment 142974View attachment 142975View attachment 142976View attachment 142977View attachment 142978
Everytime I have to post a pic I see lot of other stuff in my phone and couldn’t resist... look at John Meadows, I love him... look at that Maserati Mc12 Corsa... prettiest car ever made... look at Florentina... she s ready for work <3
TrennedOutLunatic
Banned
What do you mean by "easily"?Yes and i mean.. you see 78 C as boiling point of Ethanolsius... but I see it evaporating at 25 C in my room.. so even if Boiling Point of BA is higher than room temp, I think it could easily evaporate Like ethanol... even if we have those data’s.. maybe “evaporatingness” isn’t related to boiling point...
Put some water and ethanol into some surface and will see they ll disappear.. even if water boils at 100 C ... so, I don’t trust boiling points and will stick to cover my becker and put BA before filtering..
Benzyl alcohol needs to be heated to ~X2.5 the temperature of ethanol in order to boil.
That's called "easily"?
3 things affect the stability of substances.
Light
Oxygen
Heat
Let's talk oxygen.
Reactivity with oxygen when you apply Ethanol to a huge surface like the table,is huge.
Also the thickness of the layer applied plays a huge role.
Leave a glass of ethanol on the table. See how long it takes to evaporate. Don't even look at it because you will see no change for a loooooong time.
You close it in a bottle,reactivity with oxygen is nullified and therefore it does not evaporate anymore.
Both are air sensitive. And the surface area is the game changer.
The fact that both are classified as alcohols doesn't mean that they behave the same.
Last edited:
FR0Z3N_B0MB3RRR
New Member
Right , but alcohol closed in a bottle couldn’t evaporate because of the stopper too...What do you mean by "easily"?
Benzyl alcohol needs to be heated to ~X2.5 the temperature of ethanol in order to boil.
That's called "easily"?
3 things affect the stability of substances.
Light
Oxygen
Heat
Let's talk oxygen.
Reactivity with oxygen when you apply Ethanol to a huge surface like the table,is huge.
Also the thickness of the layer applied plays a huge role.
Leave a glass of ethanol on the table. See how long it takes to evaporate. Don't even look at it because you will see no change for a loooooong time.
You close it in a bottle,reactivity with oxygen is nullified and therefore it does not evaporate anymore.
Both are air sensitive. And the surface area is the game changer.
The fact that both are classified as alcohols doesn't mean that they behave the same.
Anyhow ok Trenned you win, but I ll cover my becker and put BA before filtering... you will never own me
View: https://youtu.be/R_dExRpUylI
TrennedOutLunatic
Banned
Yes because there is no air exposure.Right , but alcohol closed in a bottle couldn’t evaporate because of the stopper too...
Suspended in an oil solution and diluted (we use 2%),the considerations of evaporation are eliminated or are inconsiderable at 2%. The air exposure in a beaker,mixed with bb,oil,compound is inconsiderable.
Anyhow ok Trenned you win, but I ll cover my becker and put BA before filtering... you will never own me
View: https://youtu.be/R_dExRpUylI
I don't need to own you.
I just always back up my claims,otherwise I say "I've heard" ,"rumor has it that" and shit like that.
FR0Z3N_B0MB3RRR
New Member
I was joking about the own stuffYes because there is no air exposure.
Suspended in an oil solution and diluted (we use 2%),the considerations of evaporation are eliminated or are inconsiderable at 2%. The air exposure in a beaker,mixed with bb,oil,compound is inconsiderable.
I don't need to own you.
I just always back up my claims,otherwise I say "I've heard" ,"rumor has it that" and shit like that.
anyway... does oxygen breaks inter molecular bonds? Because it attract electrons ?
today I ll make an experiment.. will put 10 ml of Benzyl alcohol, put it at 50 C and see what happens...
But mainly because I always put a timer on stirring plate and leave becker on its own...
Anyway I still think BA evaporates while brewing stuff.. I ll try it right now Ahaha
TrennedOutLunatic
Banned
10 ml Raw ba is not the same thing as 2% ba in 10ml.I was joking about the own stuff
anyway... does oxygen breaks inter molecular bonds? Because it attract electrons ?
today I ll make an experiment.. will put 10 ml of Benzyl alcohol, put it at 50 C and see what happens...
But mainly because I always put a timer on stirring plate and leave becker on its own...
Anyway I still think BA evaporates while brewing stuff.. I ll try it right now Ahaha
The properties of a solution of 4 things inside is not the same as just 1 thing
TrennedOutLunatic
Banned
I know how to prove what's wrong or right in this case.
I have several ideas actually. I am having a brainstorm.
In 12 hours max I will have this ended. I will run several tests.
Either I will prove myself right or wrong. It will show.
Everything will be documented.
I have several ideas actually. I am having a brainstorm.
In 12 hours max I will have this ended. I will run several tests.
Either I will prove myself right or wrong. It will show.
Everything will be documented.
FR0Z3N_B0MB3RRR
New Member
I know how to prove what's wrong or right in this case.
I have several ideas actually. I am having a brainstorm.
In 12 hours max I will have this ended. I will run several tests.
Either I will prove myself right or wrong. It will show.
Everything will be documented.
i should measure a solution with and without BA and weight it after and before...
I forgot that fuckin beacker with 20ml BA at 50 C ... now I came to that room were I was trying that and found a thick smell of BA and base of beaker was even darkened... all BA evaporates obv... I have tears on my eyes due to that fuckin BA...
Got your point Trenned, that’s not the same, but I remembered that I cover the becker to prevent dust & co to go into it.. since I don’t have a hoodflow
TrennedOutLunatic
Banned
That won't prove anything.i should measure a solution with and without BA and weight it after and before...
The scale won't catch the difference because the amount of ba used is miniscule.
It is 0.2ml per 10ml for 2%.
except if you are running a super sensitive and expensive laboratory scale
No need to cover anything. Just close the AC and any fans.I forgot that fuckin beacker with 20ml BA at 50 C ... now I came to that room were I was trying that and found a thick smell of BA and base of beaker was even darkened... all BA evaporates obv... I have tears on my eyes due to that fuckin BA...
Got your point Trenned, that’s not the same, but I remembered that I cover the becker to prevent dust & co to go into it.. since I don’t have a hoodflow
It is filtered afterwards.
Imagine there are people using .45um and I have heard of zero problems.
I am against it except if we are talking prefiltering. I don't gamble.
Just show me a picture of how you cover it.
I need to see if for some irrelevant reasons,for once I get to the place that i will run the experiments.
FR0Z3N_B0MB3RRR
New Member
Some cellophane, and covered the hole made by thermomether... anyway, over complicating this stuff... my fault... now I’ll pin it without filtering and without BA... I don’t give a fuck anymore... actually, i wont pin a anything anymore.. I m done... I’m growing even on TRT... you all phaggots need STEROIDS to grow... I m done with that shit... look at Mike O Hearn or Simeon Panda... they re like a fuckin Porsche Carrera GT Engine... perfect... powerful and naturally aspirated...That won't prove anything.
The scale won't catch the difference because the amount of ba used is miniscule.
It is 0.2ml per 10ml for 2%.
except if you are running a super sensitive and expensive laboratory scale
No need to cover anything. Just close the AC and any fans.
It is filtered afterwards.
Imagine there are people using .45um and I have heard of zero problems.
I am against it except if we are talking prefiltering. I don't gamble.
Just show me a picture of how you cover it.
I need to see if for some irrelevant reasons,for once I get to the place that i will run the experiments.
View: https://youtu.be/VKjfkZzp9EM
I love that car... a lot...
TrennedOutLunatic
Banned
Some cellophane, and covered the hole made by thermomether... anyway, over complicating this stuff... my fault... now I’ll pin it without filtering and without BA... I don’t give a fuck anymore... actually, i wont pin a anything anymore.. I m done... I’m growing even on TRT... you all phaggots need STEROIDS to grow... I m done with that shit... look at Mike O Hearn or Simeon Panda... they re like a fuckin Porsche Carrera GT Engine... perfect... powerful and naturally aspirated...
View: https://youtu.be/VKjfkZzp9EM
I love that car... a lot...
Cellophane. Ok.
I am on my way.
FR0Z3N_B0MB3RRR
New Member
Ahahahahahahahahah need explanationsCellophane. Ok.
I am on my way.
TrennedOutLunatic
Banned
No no.Ahahahahahahahahah need explanations
The magnetic stirrer refuses to hold steady temperature and cannot do shit.
It is as if it is playing games with me.
High possibility it is broken.
No matter what,even if it is tomorrow,I am going to try again and report back 100%
I would keep trying but there is lockdown after 9 o'clock and it is 7:30 fml
If I manage to make it work right today,tomorrow I will be doing the actual experiments.
I am really fucking upset
FR0Z3N_B0MB3RRR
New Member
No no.
The magnetic stirrer refuses to hold steady temperature and cannot do shit.
It is as if it is playing games with me.
High possibility it is broken.
No matter what,even if it is tomorrow,I am going to try again and report back 100%
I would keep trying but there is lockdown after 9 o'clock and it is 7:30 fml
If I manage to make it work right today,tomorrow I will be doing the actual experiments.
I am really fucking upset
Such a shame... Electronic tools just aren't what they used to be.
Anyway, cover the becker with cellophane to prevent dust whatever is a good idea..
I proved today that some mls of BA putted in becker at 50 C evaporate very fast , you ll prove that 2% BA in oil won’t evaporate... that’s cool, since I thought different... but now I trust your argumentations
That’s total BS brotherNo no.
The magnetic stirrer refuses to hold steady temperature and cannot do shit.
It is as if it is playing games with me.
High possibility it is broken.
No matter what,even if it is tomorrow,I am going to try again and report back 100%
I would keep trying but there is lockdown after 9 o'clock and it is 7:30 fml
If I manage to make it work right today,tomorrow I will be doing the actual experiments.
I am really fucking upset
how much is the temperature fluctuating? It looks like that stirrer had a thermometer that you place in the vial to give a digital readout on the screen but on of the analog disks control the temperature-is that correct? So if you leave the dial at one of the lower notches- how much is it fluctuating? Or does it just keep heating like my electric stove does? The cheaper ones will not maintain the exact temperature but more like a range.No no.
The magnetic stirrer refuses to hold steady temperature and cannot do shit.
It is as if it is playing games with me.
High possibility it is broken.
No matter what,even if it is tomorrow,I am going to try again and report back 100%
I would keep trying but there is lockdown after 9 o'clock and it is 7:30 fml
If I manage to make it work right today,tomorrow I will be doing the actual experiments.
I am really fucking upset
Similar threads
- Replies
- 78
- Views
- 2K

