PiscesMoon
Member
FDA Approves Labeling Changes to Menopausal Hormone Therapy Products
U.S. Food and Drug Administration sent this bulletin at 02/12/2026 12:07 PM ESTFDA Approves Labeling Changes to Menopausal Hormone Therapy Products

"The U.S. Food and Drug Administration has approved drug labeling changes to six menopausal hormone therapy products, also known as hormone replacement therapy (HRT), to clarify risk considerations for these drugs. Specifically, risk statements related to cardiovascular disease, breast cancer and probable dementia were removed from the “boxed warning,” the agency’s most prominent safety-related warning.
“This decision reflects our commitment to follow the science wherever it leads and to correct course when the evidence demands it,” Health and Human Services Secretary Robert F. Kennedy, Jr. said. “By removing these boxed warnings, we ensure that women receive accurate information about hormone therapy—free from exaggeration or fear. A healthcare system worthy of public trust tells the truth, updates its guidance as science evolves, and respects women’s ability to make informed choices about their own health.”
The FDA initiated the removal of these warnings in November 2025, following a comprehensive review of the scientific literature. At the FDA’s request, 29 drug companies have submitted proposed labeling changes. This first batch of six products with approved labeling changes includes products from each of the four categories of HRT for menopausal women:
- Systemic combination therapy (estrogen and progestogen)
- Systemic estrogen-alone therapy
- Systemic progestogen-alone therapy for women with a uterus using systemic estrogen
- Topical vaginal estrogen therapy
Menopause is a normal life stage, but its symptoms can significantly reduce quality of life. Common symptoms include hot flashes and night sweats (called vasomotor symptoms or VMS); vaginal, vulvar, and urinary tract changes caused by lower estrogen levels; and osteoporosis (thinning bones), which increases fracture risk.
The FDA has approved multiple hormone therapies for moderate-to-severe hot flashes, vaginal dryness and discomfort, and preventing bone loss. In addition, randomized studies show that women who initiate HRT within 10 years of the onset of menopause (generally before age 60) have a reduction in all-cause mortality and fractures. Just a small fraction of women who could benefit from these treatments, however, are using them. In 2020, about 41 million U.S. women were ages 45–64 — yet only about 2 million women ages 46–65 received a hormone-therapy prescription.
Today’s action will allow women, working with their health care professionals, to make better-informed decisions about their treatment plan for menopause symptoms. Women are encouraged to consult the drug label, available here*, for more detailed information about the benefits and risks of these products."
* Drug Label Changes:
Menopausal Hormone Therapies with Updated Prescribing Information
(Image below shows the index of updated products.
Note that product links = .pdf files)
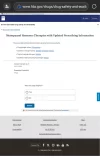
"Menopausal Hormone Therapies with Updated Prescribing Information
The prescribing information has been updated on these products.
- Progestogen alone: Prometrium
- Topical vaginal estrogen: Estring
- Systemic estrogen and progestogen: Bijuva
- Content current as of:02/12/2026"




