Chen PR, Lee K. INVITED REVIEW: Inhibitors of myostatin as methods of enhancing muscle growth and development. J Anim Sci 2016;94(8):3125-34. https://dl.sciencesocieties.org/publications/jas/abstracts/94/8/3125
With the increasing demand for affordable, high-quality meat, livestock and poultry producers must continually find ways to maximize muscle growth in their animals without compromising palatability of the meat products.
Muscle mass relies on myoblast proliferation during prenatal or prehatch stages and fiber hypertrophy through protein synthesis and nuclei donation by satellite cells after birth or hatch. Therefore, understanding the cellular and molecular mechanisms of myogenesis and muscle development is of great interest.
Myostatin is a well-known negative regulator of muscle growth and development that inhibits proliferation and differentiation in myogenic cells as well as protein synthesis in existing muscle fibers. In this review, various inhibitors of myostatin activity or signaling are examined that may be used in animal agriculture for enhancing muscle growth.
Myostatin inhibitors are relevant as potential therapies for muscle-wasting diseases and muscle weakness in humans and animals. Currently, there are no commercial myostatin inhibitors for agriculture or biomedical purposes because the safest and most effective option has yet to be identified. Further investigation of myostatin inhibitors and administration strategies may revolutionize animal production and the medical field.
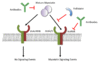
Myostatin activity is inhibited through several mechanisms.
Follistatin can directly bind to a myostatin dimer and prevent binding to activin receptor IIB (ActRIIB).
Antibodies can either bind to a myostatin dimer to prevent signaling or bind to ActRIIB to act as competitive inhibitors.
The dominant negative mutant of ActRIIB (dnActRIIB) is lacking the cytoplasmic kinase domain; therefore, the myostatin dimer can bind to the receptor but the signaling cascade does not occur.
ALK4/5 = activin receptor-like kinase 4/5.
With the increasing demand for affordable, high-quality meat, livestock and poultry producers must continually find ways to maximize muscle growth in their animals without compromising palatability of the meat products.
Muscle mass relies on myoblast proliferation during prenatal or prehatch stages and fiber hypertrophy through protein synthesis and nuclei donation by satellite cells after birth or hatch. Therefore, understanding the cellular and molecular mechanisms of myogenesis and muscle development is of great interest.
Myostatin is a well-known negative regulator of muscle growth and development that inhibits proliferation and differentiation in myogenic cells as well as protein synthesis in existing muscle fibers. In this review, various inhibitors of myostatin activity or signaling are examined that may be used in animal agriculture for enhancing muscle growth.
Myostatin inhibitors are relevant as potential therapies for muscle-wasting diseases and muscle weakness in humans and animals. Currently, there are no commercial myostatin inhibitors for agriculture or biomedical purposes because the safest and most effective option has yet to be identified. Further investigation of myostatin inhibitors and administration strategies may revolutionize animal production and the medical field.

Myostatin activity is inhibited through several mechanisms.
Follistatin can directly bind to a myostatin dimer and prevent binding to activin receptor IIB (ActRIIB).
Antibodies can either bind to a myostatin dimer to prevent signaling or bind to ActRIIB to act as competitive inhibitors.
The dominant negative mutant of ActRIIB (dnActRIIB) is lacking the cytoplasmic kinase domain; therefore, the myostatin dimer can bind to the receptor but the signaling cascade does not occur.
ALK4/5 = activin receptor-like kinase 4/5.

