Ghoul
Member
What if you got parkinsons?
You can exercise your perineum (the pee stopping muscle) to reduce any dribbling. Unfortunately a really strong perineum weakens orgasms....
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
What if you got parkinsons?
Your just like and endless supply of information. Seriously I've learned more from this thread and specifically ghoul than the last 5 years of my life.You can exercise your perineum (the pee stopping muscle) to reduce any dribbling. Unfortunately a really strong perineum weakens orgasms....
Damned if you do, damned if you don't.You can exercise your perineum (the pee stopping muscle) to reduce any dribbling. Unfortunately a really strong perineum weakens orgasms....
not prostate orgasmsUnfortunately a really strong perineum weakens orgasms....
Ugh. And on this note, I’m sticking around a little longer to see if T returns. I saw that Ray is back but many CNY festivities last until next week. So we’ll see. Doesn’t look good.not prostate orgasms
Like I said, I’ll give till 12th then I’m chalking up a 1k lossUgh. And on this note, I’m sticking around a little longer to see if T returns. I saw that Ray is back but many CNY festivities last until next week. So we’ll see. Doesn’t look good.
Looks like the 4000th page promo will never happen.
Ouch. Thats a tough one to swallow. At least it would be for me. I know a lot of you guys are straight ballers. Rolexes and filters and so on.Like I said, I’ll give till 12th then I’m chalking up a 1k loss
Def not a baller. I was reselling local so it was a gamble from the start.Ouch. Thats a tough one to swallow. At least it would be for me. I know a lot of you guys are straight ballers. Rolexes and filters and so on.
I’m on this struggle bus too. I’ve actually given up on this method, so hopefully ghoul has pointers. The things that make fluid loss marginally smaller are using a needle to back fill so fluid goes straight down without air bubbles, then slowly putting in the plunger until it’s barely secure, flipping the syringe so the fluid falls to the plunger end, then getting the air out. But it’s messy.Do any of you guys who are doing per dose filtering of peps/ hgh by back filling into slin have any pointers for how to do it without losing so much after plunger is reinserted and how to even get correct measure for lower doses. Even a 10 unit dose is difficult to accurately measure off the host 3ml syringe so provided the fluid goes to bottom of slin it can be measured that way but then for me I’m losing a lot reinserting plunger no matter how i hold the syringe and the peps won’t flow in the syringe like oils do. @SWFL-239 @Ghoul
Do any of you guys who are doing per dose filtering of peps/ hgh by back filling into slin have any pointers for how to do it without losing so much after plunger is reinserted and how to even get correct measure for lower doses. Even a 10 unit dose is difficult to accurately measure off the host 3ml syringe so provided the fluid goes to bottom of slin it can be measured that way but then for me I’m losing a lot reinserting plunger no matter how i hold the syringe and the peps won’t flow in the syringe like oils do. @SWFL-239 @Ghoul


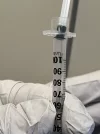

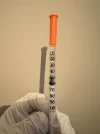
I’m on this struggle bus too. I’ve actually given up on this method, so hopefully ghoul has pointers. The things that make fluid loss marginally smaller are using a needle to back fill so fluid goes straight down without air bubbles, then slowly putting in the plunger until it’s barely secure, flipping the syringe so the fluid falls to the plunger end, then getting the air out. But it’s messy.
I’m using a 1 1/2” 18ga needle to push the fluid in and i think i finally got to the point where it’s going all the way down, its just getting the fluid to move back towards plunger so i have some air to push out is my biggest issue but ill stick with it lol. Definitely a lot of trial and error with this methodI’m on this struggle bus too. I’ve actually given up on this method, so hopefully ghoul has pointers. The things that make fluid loss marginally smaller are using a needle to back fill so fluid goes straight down without air bubbles, then slowly putting in the plunger until it’s barely secure, flipping the syringe so the fluid falls to the plunger end, then getting the air out. But it’s messy.
Really appreciate the info and thanks for adding the pics, i guess at this point for me it’s a finesse thing with the plunger. Right now i think im putting it in too much for fluid to flow back easily and the pressure is pushing out the fluid. I’m also having difficulty finding a source for the silicon free syringes so if you have one and don’t mind sharing i would appreciate it. I am storing the filter syringes upright with a bit of air between plunger and fluid to try and minimize contact.It's probobly best demonstrated by a video, but I'm not sure I'm motivated enough to make one.
It did take some trial and error, making a few messes. but I'll give you a few tips that may help.
-I use the cap from the "push" end of the insulin syringe as a place to stick the plunger. It won't "stand up", but its a sterile place to put the rubber plunger into and lay it down.
View attachment 314906
-I use a 1-1.5" large gauge needle to avoid creating bubbles, stuck deeply into the syringe barrel, angled against the barrel wall, without completely blocking off the open end of the insulin syringe. This allows the liquid to flow to the bottom of the insulin syringe easily, without getting "caught", above a bubble, as you've likely experienced.
View attachment 314907
-Once finished filing, holding both syringes in one hand I recap the 3ml filtered syringe and put it down.
-Then I stick the insulin syringe plunger in *just barely". It's not even in enough to stay in on its own. It only closes off the open end and must be held there. Obviously you don't want to push out any liquid.
View attachment 314908
-Flip the insulin syringe needle side up (still capped btw), hold the barely in plunger to keep the open end closed.
View attachment 314909
-I flick the barrel with my fingernail to move the air bubble to the top. Once it's at the top, I can push the plunger in, pushing out the air (you can still leave the needle end cap on), and set down the perfectly dosed syringe for later or immediate use.
View attachment 314910
It's easier than it sounds once you practice a few times, Even small doses are no issue, though you should really be diluting to get more than .10ml volume for any peptide.
Maybe BD will sponser a video, lol.
Also, I realize this isn't "perfect", no technique is. I am improving this, eliminating the need for backfilling for per dose filtration, supplies are more expensive using this somewhat better technique. Another thing you should aim for, at least with the 3ml syringe that'll hold peptides for an extended time, is a silicone free syringe. Silicone, though common as a syringe lubricant, in increasingly understood to be bad to inject generally, but it has a negative effect on proteins/peptides in particular.
The other possibility, if you have really good control, is just to stick a zero dead space 31g needle on the end of the filtered 3ml syringe, then remove and dispose the needle after injecting. Like a "manual" injection pen. This adds a few complications too, but nothing insurmountable. It does add the risk of overdosing though and requires a very steady hand.
lmao sorry idk why I did thatTag the gentleman who wrote about the seized package, though, not me
Thanks
Wouldn't this significantly increase chances of contamination? Opening up sterile areas of the syringe and the peptide liquid to open air? For anyone into mycology, you know it's super easy for things to get contaminated in the open air. Unless you're using a flow hood. Maybe I'm missing something, but at least the way you're demonstrating it, it seems like you're trading one risk for another.It's probobly best demonstrated by a video, but I'm not sure I'm motivated enough to make one.
It did take some trial and error, making a few messes. but I'll give you a few tips that may help.
-I use the cap from the "push" end of the insulin syringe as a place to stick the plunger. It won't "stand up", but its a sterile place to put the rubber plunger into and lay it down.
View attachment 314906
-I use a 1-1.5" large gauge needle to avoid creating bubbles, stuck deeply into the syringe barrel, angled against the barrel wall, without completely blocking off the open end of the insulin syringe. This allows the liquid to flow to the bottom of the insulin syringe easily, without getting "caught", above a bubble, as you've likely experienced.
View attachment 314907
-Once finished filing, holding both syringes in one hand I recap the 3ml filtered syringe and put it down.
-Then I stick the insulin syringe plunger in *just barely". It's not even in enough to stay in on its own. It only closes off the open end and must be held there. Obviously you don't want to push out any liquid.
View attachment 314908
-Flip the insulin syringe needle side up (still capped btw), hold the barely in plunger to keep the open end closed.
View attachment 314909
-I flick the barrel with my fingernail to move the air bubble to the top. Once it's at the top, I can push the plunger in, pushing out the air (you can still leave the needle end cap on), and set down the perfectly dosed syringe for later or immediate use.
View attachment 314910
It's easier than it sounds once you practice a few times, Even small doses are no issue, though you should really be diluting to get more than .10ml volume for any peptide.
Maybe BD will sponser a video, lol.
Also, I realize this isn't "perfect", no technique is. I am improving this, eliminating the need for backfilling for per dose filtration, supplies are more expensive using this somewhat better technique. Another thing you should aim for, at least with the 3ml syringe that'll hold peptides for an extended time, is a silicone free syringe. Silicone, though common as a syringe lubricant, in increasingly understood to be bad to inject generally, but it has a negative effect on proteins/peptides in particular.
The other possibility, if you have really good control, is just to stick a zero dead space 31g needle on the end of the filtered 3ml syringe, then remove and dispose the needle after injecting. Like a "manual" injection pen. This adds a few complications too, but nothing insurmountable. It does add the risk of overdosing though and requires a very steady hand.
Agreed.Wouldn't this significantly increase chances of contamination? Opening up sterile areas of the syringe and the peptide liquid to open air? For anyone into mycology, you know it's super easy for things to get contaminated in the open air. Unless you're using a flow hood. Maybe I'm missing something, but at least the way you're demonstrating it, it seems like you're trading one risk for another.
Really appreciate the info and thanks for adding the pics, i guess at this point for me it’s a finesse thing with the plunger. Right now i think im putting it in too much for fluid to flow back easily and the pressure is pushing out the fluid. I’m also having difficulty finding a source for the silicon free syringes so if you have one and don’t mind sharing i would appreciate it. I am storing the filter syringes upright with a bit of air between plunger and fluid to try and minimize contact.
As far as dilution and dosing goes i add as much bac water as i can. For example i have 10mg vials of both TB and BPC that are actually around 12 mg. I add 3ml of bac to both but for my desired dosing of 250-300 mcg x 2 day, i’m stuck with small number of units to dose. To dilute anymore i would have to either transfer to bigger vial or go with 5mg vials
Thanks for the help @Ghoul

You banned bruh?Tag the gentleman who wrote about the seized package, though, not me
Thanks
Agreed.
I am very anti backfilling.
I'm not worried about true contamination, but for someone who is constantly talking about filtering Peptides this makes 0 sense lol.



