Caution if you go this route. All you will get is the data and Jano won't interpret. Very tedious analysis work.
I was headed down the circular dichroism path which requires a pharma standard to compare UG sample against. Interpretation much simpler.
Also the Nb2 assay for bioactivity. Haven't found a lab for that yet. The serum GH tests are a great backstop.
At $25-30 a test it would be nice to see vendors offer double or triple credit for serum GH testing (for time and inconvenience).
I think this was fairly common before Jano testing became the norm.
Obviously it's not an infallible test, mostly because of the human factor, but it really gets to the bottom line in terms effectiveness.
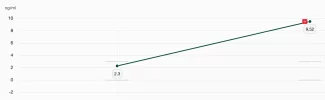
Very nice data set. From these data we can calculate 95 or 99% CI using t-score with n = 24.
Roughly 48 +/- 11.5 for 99%tile.
Hence LCL is 36.5.
10/15 × 36.5 =
24.3 ng/ml (99% lower confidence limit for 10 IU using t-score since n less than 30)
An easy confidence interval calculator for estimating a population mean from a single sample. Provides full details of workings.
www.socscistatistics.com
Thanks Ghoul. Nice floor to keep in mind for this type of test.
Impressive deep analysis, and like you said, that's the floor. We should expect better than that.
I believe Alex derived these numbers from observation of forum posted results, reinforcing the fact 10iu from a broad range of UGL and pharma brands should elicit much more than 9.5 ng/ml. 30-40ng.
The quick & dirty serum GH assay: inject 10 IU IM and get GH measured 2-3 hours later. Typical response is around 30-40 ng/mL.
For IGF1, measure before starting GH and then after a few weeks on whatever dose you're doing, then get IGF1 measured again. Time of GH injection vs time of blood draw doesn't matter. There is a pretty big range in responses.
One other clarification, modern Serum GH testing uses an antibody "sandwich" technique. It's very good at specifically measuring active rHGH only, but not perfect.
Antibodies try to dock onto specifically shaped patterns on proteins (epitopes), and when they do they activate in a way that can be detected by the analysis machine ( they become uv reactive for instance).
But these epitopes can be intact on protein fragments or misfolded proteins causing an inactive molecule to be falsely detected as bioactive.
Sandwiching requires 2 antibodies that both have to dock on separate parts of the rHGH molecule in order to "count", and if it's broken, misfolded, or oxidized it's very unlikely for both antibodies to dock.
So the TLDR is there's still a small chance of inactive rHGH giving a falsely high serum GH reading, but very unlikely to be more than a few percent, vs HPLC which counts many inactive forms (misfolded, oxidized) towards purity. Running the numbers, if 60% of an rHGH sample in a 97% pure rHGH consisted of inactive rHGH, serum GH would "see" <10% of those inactive monomers.
Also, defective rHGH that counts as "pure" in HPLC is more likely to rapidly degrade or be destroyed by the immune system once injected. A bad formulation resulting in the wrong PH can make rHGH precipitate in the injection site. So none of those reach the blood and are excluded from the serum GH number, making it an even more accurate measure of functional rHGH.