Ghoul
Member
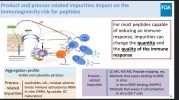
The December 2024 FDA meeting of the Compounding Pharmacy Advisory Committee is once again sounding the alarm on peptide immunogenicity risk, this time focusing in on aggregates as the primary factor, and the failure of non-pharma produced peptide formulations to prevent their development. They suggest aggregation control is the primary means by which to reduce risk of immunogenicity.*
CJC-1295, all forms, banned from compounding use by a majority vote due to concerned over genetic mutations, immunogenicity risk, and safer GH secretagogue availability,
AOD-9604, both forms, banned from compounding use by a majority vote due to immunogenicity risk, lack of safety and efficacy data.
Both may be reconsidered in the future if a manufacturer sponsors normal pharmaceutical clinical trial so safety and efficacy can be fully evaluated.


Full set of meeting slides available here:
* I suggest UGL peptide users exercise aggregate control through proper reconstitution, handling, and filtration.
CJC-1295, all forms, banned from compounding use by a majority vote due to concerned over genetic mutations, immunogenicity risk, and safer GH secretagogue availability,
AOD-9604, both forms, banned from compounding use by a majority vote due to immunogenicity risk, lack of safety and efficacy data.
Both may be reconsidered in the future if a manufacturer sponsors normal pharmaceutical clinical trial so safety and efficacy can be fully evaluated.



Full set of meeting slides available here:
* I suggest UGL peptide users exercise aggregate control through proper reconstitution, handling, and filtration.
Last edited:

