Appreciate the positive words brother!Looking good man. Patience and hard work / diet / cardio. It’s a marathon not a sprint. Keep your doses where they are and don’t worry about upping them quickly. More gear isn’t always better. The results definitely make you want to take more, I think we’ve all been in that boat but it’s a mental game to keep pushing on without doing so.
Much respect to your honesty and posting your journey.
As a fellow tradesmen myself I know the long hours and gas station food / energy drinks are always so tempting!
Keep it up brother!
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
Style variation
Guest viewing is limited
- You have a limited number of page views remaining
- 5 guest views remaining
- Register now to remove this limitation
- Already a member? Click here to login
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
First cycle primo/test/hgh bloodwork baseline and week5
- Thread starter Dirthand
- Start date
UncleBuns
Member
Why are you using such high doses? 1.6g of AAS a week and 10iu GH a day is a HUGE cycle. That is a big cycle even for a huge body builder who has run many cycles. I would do 1/2 of that. You will likely still get great results and your health markers will be in much better shape.
You are definantly right. I was just kinda pyramid my doses up just to kinda see how my body responds. I mean I didn't just start at these doses and all compounds at once I built up over the last 14 weeks. Doing labs every 5 weeks. I plan on pyramid back down over the next 8 weeks to a cruise at 22 weeks. I'm not saying it was a good way or the right way, it's just how I did it and how I am doing it and I don't regret it. I have learned a lot and definantly have a lot more to learn. I respect you guys opinions.. especially yours buns I know your gonna call like you see it. I do think I have gotten a little carried away with my doses and will start to tirate back down. I do workout hard 5 days a week and my diet is very clean. Thanks for the heart check!Why are you using such high doses? 1.6g of AAS a week and 10iu GH a day is a HUGE cycle. That is a big cycle even for a huge body builder who has run many cycles. I would do 1/2 of that. You will likely still get great results and your health markers will be in much better shape.
Peace
UncleBuns
Member
No offense but it seems excessive for your cycle history and current state. Your progress is super impressive and I'm confident you'll go much further. No point in excessive doses which will do little to further your goal but will definitely harm your health. Stick with moderation and the consistency you've already shown and you're golden. Drugs can't overcome the time it takes to reach goals but they can do damage for no benefit. You're on your way, no use in rushing. Enjoy the ride.You are definantly right. I was just kinda pyramid my doses up just to kinda see how my body responds. I mean I didn't just start at these doses and all compounds at once I built up over the last 14 weeks. Doing labs every 5 weeks. I plan on pyramid back down over the next 8 weeks to a cruise at 22 weeks. I'm not saying it was a good way or the right way, it's just how I did it and how I am doing it and I don't regret it. I have learned a lot and definantly have a lot more to learn. I respect you guys opinions.. especially yours buns I know your gonna call like you see it. I do think I have gotten a little carried away with my doses and will start to tirate back down. I do workout hard 5 days a week and my diet is very clean. Thanks for the heart check!
Peace
Thanks man I really did need the heart check on the situation!No offense but it seems excessive for your cycle history and current state. Your progress is super impressive and I'm confident you'll go much further. No point in excessive doses which will do little to further your goal but will definitely harm your health. Stick with moderation and the consistency you've already shown and you're golden. Drugs can't overcome the time it takes to reach goals but they can do damage for no benefit. You're on your way, no use in rushing. Enjoy the ride.
Nice work keeping consistent and making progress.
Var is notorious for making lipids unfavorable.
I experience unreasonable skews in lipids on oxandrolone too.
I paid out of pocket for a CT scan of arteries and calcium score as the issue with high lipids is plaque accumulation and potential blockage.
I did the CT scan and echo for like $250 from prime imaging. If you were super worried about the lipids that may be a piece of mind for you.
Not sure about the kidney tests tho if you were properly hydrated with those labs. That over my head.
Var is notorious for making lipids unfavorable.
I experience unreasonable skews in lipids on oxandrolone too.
I paid out of pocket for a CT scan of arteries and calcium score as the issue with high lipids is plaque accumulation and potential blockage.
I did the CT scan and echo for like $250 from prime imaging. If you were super worried about the lipids that may be a piece of mind for you.
Not sure about the kidney tests tho if you were properly hydrated with those labs. That over my head.
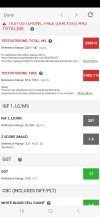
View attachment 278597does this seem right to have an igf-1 of 287 on 10iu of growth a day??previously 300 on 5iu
I think many would say that is a poor response.
Are you getting tested GH? A lot goes into the GH to igf conversion I won’t pretend to know.
My results below are on goodlyfe Gh at 2iu and the previous result 3.5iu
Attachments
Eddie.
Member
I haven't researched much about GH and haven't used obviously but from what i hear from various podcast of known gurus that many like to hate here on meso, serum IGF doesn't predict the purity and how legit the GH is. If i'm not mistaken even Chase Irons with 18iu of pharma doesn't have a impressive IGF number. IGF is a multifactoral thing from what i get, but take this with a grain of salt..
This is true. Hgh serum test though can at least give you an idea if there is any hgh in there and a rough estimate of how much but not accurateI haven't researched much about GH and haven't used obviously but from what i hear from various podcast of known gurus that many like to hate here on meso, serum IGF doesn't predict the purity and how legit the GH is. If i'm not mistaken even Chase Irons with 18iu of pharma doesn't have a impressive IGF number. IGF is a multifactoral thing from what i get, but take this with a grain of salt..
R
readalot
Guest
How useful are serum IGF-I measurements for managing GH replacement therapy in adults and children? - Pituitary
The optimal dosing of growth hormone (GH) therapy is challenging due to high inter-individual variability in subcutaneous GH absorption and sensitivity to the drug. Optimal dosing would maximize patient gains in height, body composition, and metabolic outcomes while minimizing GH adverse events...
An important limitation to current IGF-I assay procedures is the potential for intra-patient variability in assay results, at least as has been shown in healthy subjects [17]. For example, in one study, IGF-I levels in healthy subjects age 50–90 years varied up to 30% between two visits made in a 2-weeks period, resulting in a change in IGF-I quartile in 40% of the subjects[32]. In this study, the intra-patient variability exceeded the assay imprecision, suggesting biological within-subject variability of IGF-I contributed to the differences over time in healthy subjects [17, 32]. However, it was stated that variability in IGF-I may have in part been due to handling, storage, or shipping [32].

Population Pharmacokinetics and Pharmacodynamics of Once-Daily Growth Hormone Norditropin® in Children and Adults - Clinical Pharmacokinetics
Background and Objective Once-daily injectable recombinant human growth hormone (GH) formulations (e.g. Norditropin®; Novo Nordisk A/S) are used to treat GH deficiency in children and adults, with much of the therapeutic effect mediated via the insulin-like growth factor-I (IGF-I) response...
Fig. 4

Population Pharmacokinetics and Pharmacodynamics of Once-Daily Growth Hormone Norditropin® in Children and Adults - Clinical Pharmacokinetics
Background and Objective Once-daily injectable recombinant human growth hormone (GH) formulations (e.g. Norditropin®; Novo Nordisk A/S) are used to treat GH deficiency in children and adults, with much of the therapeutic effect mediated via the insulin-like growth factor-I (IGF-I) response...
Last edited by a moderator:
Shivathedestroyer
Member
Brother… when you first started this thread I told you this was going to happen.Well I just get the results on some new labs taken at week13. My lipids are extremely horrible.. I did cycle anavar for 4 weeks but been off for 2 weeks. I am starting crestor at20mg a day and see if that helps. Also I stopped doing cardio a couple weeks ago but will start back.
At time of lab I was at
Testc. 600mg
Primo 550
Npp. 420
Pin daily
Does anyone have any thoughts besides " stop at once"View attachment 278365View attachment 278366View attachment 278367View attachment 278368View attachment 278369View attachment 278370View attachment 278371
I was not being a smart ass when I said “I would not blast any gear if my bloods looked like that”
These bloods are fucking awful dude, I don’t agree with the person above saying that these are normal values for people running big cycles.
I’ve run some big cycles and coached guys who have run big cycles and and the biggest priority is keeping health markers in check and this is definitely way worse blood work than I’ve seen.
Your cystatin c / egfr is very alarming and when you started this thread and I told you that your kidney values sucked you said they weren’t that bad. Your creatine was high and your bun was high and your egfr was tanked.
You doubled down and cranked your dosages way up, what did you think was going to happen?
This is just purely irresponsible in ever single regard. Your health was not in a good spot to start this cycle let alone increase your doses by 2-3x
I agree with readlot, Godspeed brother
Edit: and I see you decided to throw some orals in the mix too.
Do you just have total disregard for your own health and well-being, serious question.
Last edited:
Hey I definantly respect you and your opinion. You seem like a stand up guy! My health markers are not that great but actually there has been some small improvement in all ares except for my lipids. I'm not looking for perfect or great. I'm looking for doable. I totally disagree that it's an end of the world situation! I am a man and I'm not gonna tuck tail and run cause things are warming up a little, I'm going to calculate the risk vs reward . I am in the process of enlisting some professional help from this point forward. Let's just calm down a little bit brother. Besides I'm perfectly OK with my time when it's my time!Brother… when you first started this thread I told you this was going to happen.
I was not being a smart ass when I said “I would not blast any gear if my bloods looked like that”
These bloods are fucking awful dude, I don’t agree with the person above saying that these are normal values for people running big cycles.
I’ve run some big cycles and coached guys who have run big cycles and and the biggest priority is keeping health markers in check and this is definitely way worse blood work than I’ve seen.
Your cystatin c / egfr is very alarming and when you started this thread and I told you that your kidney values sucked you said they weren’t that bad. Your creatine was high and your bun was high and your egfr was tanked.
You doubled down and cranked your dosages way up, what did you think was going to happen?
This is just purely irresponsible in ever single regard. Your health was not in a good spot to start this cycle let alone increase your doses by 2-3x
I agree with readlot, Godspeed brother
Edit: and I see you decided to throw some orals in the mix too.
Do you just have total disregard for your own health and well-being, serious question.
Peace
And on another note .... I am a man so if you can talk to me with a little more respect just keep your fucking mouth shut!
Fattyone
Member
How’s your lab work?
Similar threads
- Replies
- 0
- Views
- 52