Type-IIx
Member
Interesting question, from where do oxymetholone's ostensible "gynecomastic" sides arise?
1. Tendency to increase 17-OHP, a "weak" progestin
2. Potential ER-α activation potency of 17α-methyl-5α-androstane-3α,17β-diol (also a methyltestosterone metabolite)
3. Potential for MR agonism?
4. Potential activation of RAAS?
On 1: Debatable. Progestins may induce gynecomastia (or more likely, perhaps lactation) by several mechanisms (by increasing sensitivity to estrogens, directly contributing to HPG axis suppression/secondary hypogonadism, estrogens up-regulate PR synthesis, some evidence of PR in some male breast cells, etc.) Conversely, Camerino cites Junkmann and Suchowsky, 1962 that "showed no gestagenic effect" at therapeutic doses. Oxymetholone possesses no direct PR binding: moderately to AR, weakly to ER-α (weaker than oxandrolone and stanozolol) & ER-β in mammalian reporter gene bioassay (Houtman). Likely irrelevance of model (H295R cells; adrenocortical carcinoma cells). This would seem to be a weak or disputable hypothesis...
On 2: A similar testosterone metabolite, 5α-androstane-3β,17β-diol, is a known potent activator of ER-β, and it lacks the 17α-methyl group present in this oxymetholone metabolite (which serves to prolong any anabolic effects via hepatic metabolism, and hinders aromatization of the A-ring; its 17β-OH group would serve to form a strong bond with the C-terminus/carboxy- group of the AR; saturated A-ring & 5α-androstane [hydrophobic backbone; flatness] structure confers considerable anabolic potency; α-oriented 3-OH provides electrochemical properties conducive to eventually binding to the AR's ligand-binding domain [though perhaps not as readily as a 3-keto])). Cursorily, 17α-methyl-5α-androstane-3α,17β-diol would appear to be a good candidate for AR activity.
On 3: Speculatively, oxymetholone may act as an agonist to the MR: increasing aldosterone, thereby increasing sodium retention in the renal tubules. In this sense, Anadrol may act oppositely of Tren in this regard (as methyltrienolone has been demonstrated to act as an antagonist at the MR).
On 4: Speculatively, there is some data that androgens interact with/activate the renin-aldosterone angiotensin system (RAAS). The RAAS regulates water and electrolyte balance, connective tissue cell growth, and the metabolism of loose and dense connective tissue or tissue repair, as well as bone metabolism. Pathologically, it increases vascoconstriction, cardiac hypertrophy, fibrosis (i.e., chronic activation may cause myocardial infarction, fibrosis of the liver).
... Let's explore possibility number 2 more deeply:
Oxymetholone itself is a very weak AR ligand
Fragkaki found Anadrol's AR Κᵢ (dissociation constant) to be so high/RBA too low for measurement! It is proposed that there must be an indirect mechanism of action (e.g., via biotransformation to active compound). This is also consistent with Saartok, demonstrating immeasurably low binding affinity in rat & rabbit muscle, human SHBG; but higher binding affinities for some DHT metabolites bearing some structural similarity to oxymetholone's principal active metabolite...
Metabolism
Schanzer hypothesizes that oxymetholone likely acts as a prohormone to its more active metabolites that cursorily seem to possess AR activity.
17α-methyl-5α-androstane-3α,17β-diol (Oxymetholone's active metabolite)
- Decent bioavailability (0.55)
- Druglikeness profound
- GI absorption high
- BBB permeant
- Moderately soluble
- Lipophilicity: Consensus Log Po/w 3.55
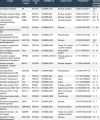
(Truncated, Summary)
The data is interesting: it does appear that oxymetholone likely acts as a prohormone to this active compound. If anyone has any data quantifying 17α-methyl-5α-androstane-3α,17β-diol's potency to activate AR, ER, GR, MR in, for example, a luciferase assay, I would be most interested!
1. Tendency to increase 17-OHP, a "weak" progestin
2. Potential ER-α activation potency of 17α-methyl-5α-androstane-3α,17β-diol (also a methyltestosterone metabolite)
3. Potential for MR agonism?
4. Potential activation of RAAS?
On 1: Debatable. Progestins may induce gynecomastia (or more likely, perhaps lactation) by several mechanisms (by increasing sensitivity to estrogens, directly contributing to HPG axis suppression/secondary hypogonadism, estrogens up-regulate PR synthesis, some evidence of PR in some male breast cells, etc.) Conversely, Camerino cites Junkmann and Suchowsky, 1962 that "showed no gestagenic effect" at therapeutic doses. Oxymetholone possesses no direct PR binding: moderately to AR, weakly to ER-α (weaker than oxandrolone and stanozolol) & ER-β in mammalian reporter gene bioassay (Houtman). Likely irrelevance of model (H295R cells; adrenocortical carcinoma cells). This would seem to be a weak or disputable hypothesis...
On 2: A similar testosterone metabolite, 5α-androstane-3β,17β-diol, is a known potent activator of ER-β, and it lacks the 17α-methyl group present in this oxymetholone metabolite (which serves to prolong any anabolic effects via hepatic metabolism, and hinders aromatization of the A-ring; its 17β-OH group would serve to form a strong bond with the C-terminus/carboxy- group of the AR; saturated A-ring & 5α-androstane [hydrophobic backbone; flatness] structure confers considerable anabolic potency; α-oriented 3-OH provides electrochemical properties conducive to eventually binding to the AR's ligand-binding domain [though perhaps not as readily as a 3-keto])). Cursorily, 17α-methyl-5α-androstane-3α,17β-diol would appear to be a good candidate for AR activity.
On 3: Speculatively, oxymetholone may act as an agonist to the MR: increasing aldosterone, thereby increasing sodium retention in the renal tubules. In this sense, Anadrol may act oppositely of Tren in this regard (as methyltrienolone has been demonstrated to act as an antagonist at the MR).
On 4: Speculatively, there is some data that androgens interact with/activate the renin-aldosterone angiotensin system (RAAS). The RAAS regulates water and electrolyte balance, connective tissue cell growth, and the metabolism of loose and dense connective tissue or tissue repair, as well as bone metabolism. Pathologically, it increases vascoconstriction, cardiac hypertrophy, fibrosis (i.e., chronic activation may cause myocardial infarction, fibrosis of the liver).
... Let's explore possibility number 2 more deeply:
Oxymetholone itself is a very weak AR ligand
Fragkaki found Anadrol's AR Κᵢ (dissociation constant) to be so high/RBA too low for measurement! It is proposed that there must be an indirect mechanism of action (e.g., via biotransformation to active compound). This is also consistent with Saartok, demonstrating immeasurably low binding affinity in rat & rabbit muscle, human SHBG; but higher binding affinities for some DHT metabolites bearing some structural similarity to oxymetholone's principal active metabolite...
Metabolism
Schanzer hypothesizes that oxymetholone likely acts as a prohormone to its more active metabolites that cursorily seem to possess AR activity.
17α-methyl-5α-androstane-3α,17β-diol (Oxymetholone's active metabolite)
- Decent bioavailability (0.55)
- Druglikeness profound
- GI absorption high
- BBB permeant
- Moderately soluble
- Lipophilicity: Consensus Log Po/w 3.55

(Truncated, Summary)
The data is interesting: it does appear that oxymetholone likely acts as a prohormone to this active compound. If anyone has any data quantifying 17α-methyl-5α-androstane-3α,17β-diol's potency to activate AR, ER, GR, MR in, for example, a luciferase assay, I would be most interested!


