McFarlane C, Vajjala A, Arigela H, et al. Negative Auto-Regulation of Myostatin Expression is Mediated by Smad3 and MicroRNA-27. PLoS One 2014;9(1):e87687. PLOS ONE: Negative Auto-Regulation of Myostatin Expression is Mediated by Smad3 and MicroRNA-27
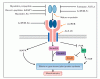
Growth factors, such as myostatin (Mstn), play an important role in regulating post-natal myogenesis. In fact, loss of Mstn has been shown to result in increased post-natal muscle growth through enhanced satellite cell functionality; while elevated levels of Mstn result in dramatic skeletal muscle wasting through a mechanism involving reduced protein synthesis and increased ubiquitin-mediated protein degradation.
Here we show that miR-27a/b plays an important role in feed back auto-regulation of Mstn and thus regulation of post-natal myogenesis. Sequence analysis of Mstn 3' UTR showed a single highly conserved miR-27a/b binding site and increased expression of miR-27a/b was correlated with decreased expression of Mstn and vice versa both in vitro and in mice in vivo.
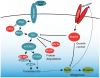
Moreover, we also show that Mstn gene expression was regulated by miR-27a/b. Treatment with miR-27a/b-specific AntagomiRs resulted in increased Mstn expression, reduced myoblast proliferation, impaired satellite cell activation and induction of skeletal muscle atrophy that was rescued upon either blockade of, or complete absence of, Mstn.
Consistent with this, miR-27a over expression resulted in reduced Mstn expression, skeletal muscle hypertrophy and an increase in the number of activated satellite cells, all features consistent with impaired Mstn function.
Loss of Smad3 was associated with increased levels of Mstn, concomitant with decreased miR-27a/b expression, which is consistent with impaired satellite cell function and muscular atrophy previously reported in Smad3-null mice. Interestingly, treatment with Mstn resulted in increased miR-27a/b expression, which was shown to be dependent on the activity of Smad3.
These data highlight a novel auto-regulatory mechanism in which Mstn, via Smad3 signaling, regulates miR-27a/b and in turn its own expression. In support, Mstn-mediated inhibition of Mstn 3' UTR reporter activity was reversed upon miR-27a/b-specific AntagomiR transfection. Therefore, miR-27a/b, through negatively regulating Mstn, plays a role in promoting satellite cell activation, myoblast proliferation and preventing muscle wasting.