[2004] Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child
[Has there been additional follow-up on the child?]
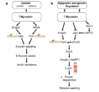
The function of myostatin appears to be conserved across species, since mutations in the myostatin gene have been shown to be responsible for the “double-muscling” phenotype in cattle. The phenotypes of mice and cattle lacking myostatin and the high degree of sequence conservation of the predicted myostatin protein in many mammalian species have raised the possibility that myostatin may help regulate muscle growth in humans.
We report the identification of a myostatin mutation in a child with muscle hypertrophy, thereby providing strong evidence that myostatin does play an important role in regulating muscle mass in humans.
…
These results strongly indicate that our patient has a loss-of-function mutation in the myostatin gene, thus suggesting that the inactivation of myostatin has similar effects in humans, mice, and cattle.
So far, we have not observed any health problems in the patient. Since myostatin is also expressed in the heart, we have closely monitored our patient's cardiac function but have not yet detected any signs of cardiomyopathy or a conduction disturbance.
However, at 4.5 years of age, our patient is still too young for such abnormalities to be ruled out definitively. Our results suggest the possibility that muscle bulk and strength could be therapeutically increased by the inactivation of myostatin in patients with muscle-wasting conditions.
Schuelke M, Wagner KR, Stolz LE, et al. Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. New England Journal of Medicine 2004;350(26):2682-8. http://www.nejm.org/doi/full/10.1056/NEJMoa040933
[Has there been additional follow-up on the child?]
The function of myostatin appears to be conserved across species, since mutations in the myostatin gene have been shown to be responsible for the “double-muscling” phenotype in cattle. The phenotypes of mice and cattle lacking myostatin and the high degree of sequence conservation of the predicted myostatin protein in many mammalian species have raised the possibility that myostatin may help regulate muscle growth in humans.
We report the identification of a myostatin mutation in a child with muscle hypertrophy, thereby providing strong evidence that myostatin does play an important role in regulating muscle mass in humans.
…
These results strongly indicate that our patient has a loss-of-function mutation in the myostatin gene, thus suggesting that the inactivation of myostatin has similar effects in humans, mice, and cattle.
So far, we have not observed any health problems in the patient. Since myostatin is also expressed in the heart, we have closely monitored our patient's cardiac function but have not yet detected any signs of cardiomyopathy or a conduction disturbance.
However, at 4.5 years of age, our patient is still too young for such abnormalities to be ruled out definitively. Our results suggest the possibility that muscle bulk and strength could be therapeutically increased by the inactivation of myostatin in patients with muscle-wasting conditions.
Schuelke M, Wagner KR, Stolz LE, et al. Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. New England Journal of Medicine 2004;350(26):2682-8. http://www.nejm.org/doi/full/10.1056/NEJMoa040933