So, I'm not too good on assays, so my apologies. Most won't be interested, but I feel like it ought to be shared with community.
As some of you, who have read my thread know, I've implemented even more strict method for testing HGH this year: Janoshik Analytical laboratory testing services
Not because my old method was bad - it was already more strict than regulating authorities require in Europe, US and rest of the civilized world as well.
My aim was purely scientific - I am, to put it that way - jerking myself off to the fact that I am able test stuff more precisely than was even published by respected researchers. I felt like providing tests much more sensitive to impurities and GH degradation products can bring more information to everybody. It can provide better measuring stick for the compound that people are putting into their bodies, it can help explain why the sides are more pronounced with one product and less with other. I also hoped that more sensitive testing would incentivize generic manufacturers to provide higher quality products. Many of you had witnessed what market was like mere 5 years ago and how testing has transformed it for the better. Now I saw this as a chance to improve my work and, in a way to improve the world for everybody involved in this kind of stuff as well.
Unfortunately, it's less than 2 weeks since I've started (after more than a month of warning beforehand about method change in my email signature) and I'm already getting MASSIVE pushback. Some of it is in my email, discord, but you can already see vendors questioning my methods over here as well. There have been claims that my method is wrong, for some vague reason, or that I am screwing something up by setting a temperature wrong, or that my method is so strict, that no hormone will ever pass it.
So I have, yet again ordered a costly EU Pharma GH standard to disprove that, despite having the method standardized to it less than a month ago.
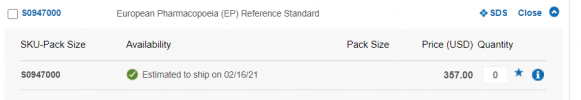

The official data for the HGH standard, which was delivered to me on ice a few hours ago are available here:
https://crs.edqm.eu/db/4DCGI/db/4DCGI/leaflet?leaflet=S0947000_3

Now, what did the new wrong method determine?

Apparently, the logic that the method is wrong, the temperature decreases the purity to less than real purity etc is not, for some reason, working on a sample purchased directly from the international authority on quality of medicines:

This also doesn't really confirm the fact that the method by itself produces severely underdosed results...
As some of you, who have read my thread know, I've implemented even more strict method for testing HGH this year: Janoshik Analytical laboratory testing services
Not because my old method was bad - it was already more strict than regulating authorities require in Europe, US and rest of the civilized world as well.
My aim was purely scientific - I am, to put it that way - jerking myself off to the fact that I am able test stuff more precisely than was even published by respected researchers. I felt like providing tests much more sensitive to impurities and GH degradation products can bring more information to everybody. It can provide better measuring stick for the compound that people are putting into their bodies, it can help explain why the sides are more pronounced with one product and less with other. I also hoped that more sensitive testing would incentivize generic manufacturers to provide higher quality products. Many of you had witnessed what market was like mere 5 years ago and how testing has transformed it for the better. Now I saw this as a chance to improve my work and, in a way to improve the world for everybody involved in this kind of stuff as well.
Unfortunately, it's less than 2 weeks since I've started (after more than a month of warning beforehand about method change in my email signature) and I'm already getting MASSIVE pushback. Some of it is in my email, discord, but you can already see vendors questioning my methods over here as well. There have been claims that my method is wrong, for some vague reason, or that I am screwing something up by setting a temperature wrong, or that my method is so strict, that no hormone will ever pass it.
So I have, yet again ordered a costly EU Pharma GH standard to disprove that, despite having the method standardized to it less than a month ago.


The official data for the HGH standard, which was delivered to me on ice a few hours ago are available here:
https://crs.edqm.eu/db/4DCGI/db/4DCGI/leaflet?leaflet=S0947000_3

Now, what did the new wrong method determine?

Apparently, the logic that the method is wrong, the temperature decreases the purity to less than real purity etc is not, for some reason, working on a sample purchased directly from the international authority on quality of medicines:

This also doesn't really confirm the fact that the method by itself produces severely underdosed results...


