Yes, I know what you mean about these redundancies.
Certainly, I think that it's more useful to group AAS into aromatizable vs. nonaromatizable than this broscience model.
It's even more useful to learn the unique biological effects particular to each AAS to understand which features can combine favorably. Favorable combinations can be grouped into those that are synergistic (greater than additive; 1 + 1 > 2), complementary (opposite, such that combination yields a "middling out" of behavior; 1 + -1 = 0), or merely additive (everything else; 1 + 1 = 2).
Synergistic combinations are those that bring about a biological effect (e.g., muscle accrual) that is the result of multiple mechanisms (e.g., anticatabolism & protein synthesis, etc.) and/or pathways (e.g., MuRF1 & atrogin-1 vs. GR expression, WISP-2 vs. IGF-IEb activity, etc.) by
different mechanisms and/or pathways, resulting in a greater than additive effects.
* Total AAS dose does not reflect effects where synergistic combinations are used.
* A higher # of AAS used, if synergistic, would be expected to be
less harmful & healthier than just ramping up total AAS dose of merely additive AAS combinations where it is used to reduce total AAS dose.
* Indeed, that is borne out by the HAARLEM trial data. There was a positive correlation between AAS weekly dose & LV mass, intraventricular end-diastolic septal thickness, and left ventricular end-diastolic posterior wall thickness & AAS dose was negatively correlated with E/e' septal. The # of AAS used was negatively associated (so more compounds means lower values of) left ventricular end-diastolic volume 3D & left ventricular end-systolic volume 3D (mL) post-cycle. Conversely, combination of rhGH & AAS resulted in higher eft ventricular end-diastolic volume 3D & left ventricular end-systolic volume 3D (mL) post-cycle.
Complementary combinations are those for which each effect acts in opposition to the other, such that their combination results in a "middling out" of behavior. For example, aromatizable & nonaromatizable androgen, combined appropriately, will result in neither net antiestrogenic nor estrogenic effects, such that tolerability is maintained. For example, combination of AAS to modulate the comfort of joints ("dry" + "wet" = robust).
Additive combinations are the remainder - everything else. These are the result of similar effects between AAS, acting via the
same mechanisms and/or pathways. Total dose (mg/week) reflects biological effects of combinations of AAS whose features are basically identical or functionally equivalent, and is a rough proxy for systemic effects after factoring in AR potency.
Important Factors that Affect Total AAS Dose (mg/week) as a Proxy for Systemic or Tissue-Level Effects
Appropriateness of mg/week as a unit of measure
Rather than weekly dose as mg/week,
dose as time × fAUC, e.g., in units 168 h fAUC (nmol h/L), more adequately describes biological effects.
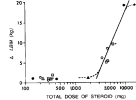
The dose/response curve (total AAS dose vs. LBM)
View attachment 261067
If AR is the sole mode of AAS action, AAS logarithmic dose/response curve should be S-shaped meaning that this dose/response would be plotted as a curve flat at both high & low doses & approximately linear at moderate doses... but for doses 100 - 1,000 mg/wk, the graph remains linear (the
assumed nonlinear portion indicated by an asterisk in the above graph was indicated by a comment that this was not actually shown by the results).
AR Regulation
In skeletal muscle, AR density is ~ 3 nM/kg. A mere 2.5 mg oxandrolone tablet provides ~ 8,000 nM androgen. Since the AR must form dimers (before translation of AR-associated mRNA) & together bind 1 mol AAS, then if, hypothetically, 100 mg T binds 71% AR, then the square of the % reflects AR activation (i.e., 50% activation).
But this is still insufficient to explain the difference between this AAS vs. LBM dose/response curve vis-à-vis different combinations ("stacks") of AAS.
Factors that regulate AR include:
* AAS dose (increasing doses of AAS increases the # of AR in skeletal muscle)
* Increased translational capacity (increased AR mRNA synthesis by increased ribosomal biogenesis)
Non-AR Mechanisms
These include, nonexhaustively:
* tethering to other transcription factors thereby influencing expression of genes without response elements in their promoters
* engaging signal transduction pathways (e.g., PI3K/Akt, IGF-I, etc.) including activation of protein kinases, thereby modulating cellular responses to androgens
* cell surface membrane-bound receptors (e.g., GPCRs) that typically modulate, e.g., Ca²⁺ uptake
*
non-ligand-dependent mechanisms
Illustration of IGF-I-mediated anabolism (a non-ligand-dependent mechanism)
Consider the non-ligand-dependent IGF-I-mediated anabolism of androgen (e.g., Testosterone), due in (small) part to aromatization and in (large) part to local muscle activity of IGF-I isoforms.
Mediated centrally (lack of AR in CNS reduces serum IGF-I) & by aromatization, Testosterone increases GH secretion, thereby increasing sytemic/circulating IGF-I (liver-secreted). This circulating GH & IGF-I plays only a minor role in AAS anabolism. Local IGF-I activity in skeletal muscle (mIGF-I) is more important.
* Testosterone increases muscle IGF-I activity more than Trenbolone, but Trenbolone increases satellite cell responsiveness to muscle IGF-I dramatically (doing more with less).
Human skeletal muscle expresses 2 IGF-I splice variants:
* IGF-IEa (similar to systemic/circulating IGF-I, liver-secreted); increases myoblast differentiation to myotubes (lesser effect on proliferation)
* IGF-IEc (MGF): mechanosensitive muscular IGF-I isoform; stimulates proliferation and inhibits terminal differentiation.
IGF-I stimulation of muscle mass relies on increased MPS (muscle protein synthesis) & myogenesis (muscle regeneration, as opposed to remodeling) & decreased proteolysis (protein breakdown) & apoptosis (cell death).
________________
Can you see how this post describes briefly, but sufficiently, some reasons that combinations of testosterone, trenbolone, and resistance training, are predictably synergistic (greater than additive) in muscle anabolism?