Ghoul
Member
I just finished listening to the October 29th meeting of the FDA Pharmacy Compounder Advisory Committee meeting.
The FDA is increasingly focusing on the threat of immunogenicity from peptide use, and beginning to quantify the differences between pharma and compounder formulations, which highlights just how much worse our UGL peptides are likely to be in this regard than the original pharma products they're copying.
TLDR: Filtering your peptide will at worst, do nothing, and at best will reduce your exposure to some unknown, potentially serious adverse health effect that may not manifest itself for many years. Also, take steps to minimize aggregation, the easiest of which is using the appropriate dilution ratio for the peptide you're reconstituting. I have personally noticed a significant reduction in site reaction/PIP after filtering the peptides that induced those effects.
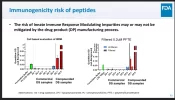
-In the example they gave, compounder produced peptide formulations produced immune reactions 100 to 1000 times stronger than pharma produced peptides.
-The types of immune response may be entirely different than the original pharma drugs due to unique impurities not present in the pharma product. This presents entirely unknown, and potentially much more serious risks than what is found during immunogenicity studies of pharma products.
-The formation of peptide aggregates, largely a function of how the peptide is reconstituted and handled, is seen as a primary cause of immunogenicity,
-In at least one case, .2um filtration reduced the immunogenic response of a compounded peptide by over 80%. Chart is below.



View: https://www.youtube.com/live/wDqrmuOOdBI
The FDA is increasingly focusing on the threat of immunogenicity from peptide use, and beginning to quantify the differences between pharma and compounder formulations, which highlights just how much worse our UGL peptides are likely to be in this regard than the original pharma products they're copying.
TLDR: Filtering your peptide will at worst, do nothing, and at best will reduce your exposure to some unknown, potentially serious adverse health effect that may not manifest itself for many years. Also, take steps to minimize aggregation, the easiest of which is using the appropriate dilution ratio for the peptide you're reconstituting. I have personally noticed a significant reduction in site reaction/PIP after filtering the peptides that induced those effects.
-In the example they gave, compounder produced peptide formulations produced immune reactions 100 to 1000 times stronger than pharma produced peptides.
-The types of immune response may be entirely different than the original pharma drugs due to unique impurities not present in the pharma product. This presents entirely unknown, and potentially much more serious risks than what is found during immunogenicity studies of pharma products.
-The formation of peptide aggregates, largely a function of how the peptide is reconstituted and handled, is seen as a primary cause of immunogenicity,
-In at least one case, .2um filtration reduced the immunogenic response of a compounded peptide by over 80%. Chart is below.




View: https://www.youtube.com/live/wDqrmuOOdBI
Last edited:




