Ghoul
Member
Thx for reviewing those key pts.
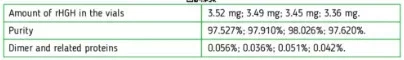
Small point: Technically, since (I assume) Jano is basing purity relative to the initial vial sample mass, he doesn't really "miss" any impurities (purity = HGH found / sample mass). We just can't say much about the nature of all those impurities based on the dimer content, although lower dimer would hopefully have some relation to immunogenicity, and good lab practice... So his results do reflect what you're injecting - he just isn't telling us much about what's in there other than the HGH and "dimers" making it thru 0.2 µm filter.
No, it's not compared to the initial vial sample mass.
The vial is reconstituted with X amount of water, a sample is withdrawn, filtered, injected into the HPLC column, separated into the various components, and the total area under the peaks of all components is compared to the area under the peak that identifies the target peptide to determine purity.
Only the post-filtration peptide sample is analyzed.
Researchers have the advantage of knowing exactly how much peptide was put into a vial, and can extrapolate how much was lost in the filter based on the amount of "missing" mass, and sometimes use that to estimate how much aggregation has taken place.