Re: Labs say low free T but high Bioavailable T. Why?
Recent findings reinforce the active role of SHBG-bound androgens in androgen action and question the validity of the free hormone hypothesis, which asserts that SHBG-bound T is a passive buffer for circulating free T. The sophisticated-sounding free hormone hypothesis, largely a product of outmoded 1970s pharmacological theory of drug interactions, has become a rarely challenged quasi-axiomatic belief in endocrinology.
The free hormone hypothesis assumes unbound hormone is more readily diffusible into tissues and thereby bioactive. This overlooks the equal likelihood that such freely diffusible hormone is also more likely to undergo inactivating metabolism so that the net balance between bioactivity and inactivation remain impossible to determine by theory. It makes many other assumptions (binding equilibria are completed during capillary transit, all capillaries operate similarly, T binding affinity for SHBG is invariant).
Empirically, the proliferation of calculational formulae for free or bioavailable T adds a further layer of inaccuracies adopting wrong stoichiometry, inaccurate SHBG binding affinity, and substitute an immunoassay for a SHBG binding assay, leading to limited validation and poor performance in large-scale usage. [Handelsman DJ. Update in Andrology. J Clin Endocrinol Metab 2007;92(12):4505-11.
Update in Andrology -- Handelsman 92 (12): 4505 -- Journal of Clinical Endocrinology & Metabolism ]
For some background, read the following. Lin BC, Scanlan TS.
Few Things in Life are “Free”: Cellular Uptake of Steroid Hormones by an Active Transport Mechanism. Molecular Interventions 2005;5(6):338-40.
Conventional dogma holds that steroid hormones traverse cell membranes passively, owing to their lipophilic nature. The recently characterized protein megalin, however, functions as a transport protein on cell surfaces to carry steroids across the plasma membrane. Upon hydrolysis of steroid-associated binding globulins in lysosomes, free hormone is liberated and may exert its effects in the cell. Megalin-independent mechanisms of steroid uptake are likely important too, as the phenotypes of megalin-deficient mice do not completely mimic the phenotypes of androgen receptor– or estrogen receptor–null mice.
Steroid hormones participate in the regulation of normal vertebrate homeostasis, development, and reproduction. The best understood mechanism of steroid action is for these signaling molecules to enter target cells and bind to their cognate intracellular receptors that, subsequently, regulate the transcription of corresponding steroid responsive genes. Defects in these signaling pathways can lead to a variety of endocrine and neoplastic disorders.
Most circulating steroids are bound to carrier proteins that deliver these hormones to their target cells. Upon reaching their destination, steroids are released and, by virtue of their small size and lipophilic nature, are believed to traverse the plasma membrane by free diffusion to carry out their regulatory effects inside the cell. Thus, the free hormone hypothesis states that the biological activity of a particular hormone is only affected by its free (protein unbound) concentration, rather than its protein-bound concentration in plasma(1).
Recent findings refute the long-held notion that lipophilic hormones, such as androgens and estrogens, solely diffuse into cells by a free, non-specific mechanism. Megalin (2, 3), a member of the low density lipoprotein receptor superfamily of endocytic proteins, has been identified as an important facilitator of steroid entry into cells. Previous work by Nykjaer and colleagues (4) has demonstrated the existence of megalin-dependent endocytic pathways for tissue specific uptake of complexed vitamin D [i.e., 25-(OH) vitamin D3 bound to vitamin D binding protein (DBP)], suggesting that megalin may be important in maintaining steroid hormone balance in mammals. Specifically, they demonstrated that megalin knockout (KO) mice were unable to resorb the vitamin into the epithelial cells of the renal proximal tubules, where the receptor is normally expressed. As a result, these megalin-null animals developed bone calcification defects, likely owing to the inability of 25-(OH) vitamin D3 to be converted to the active vitamin D receptor ligand, 1,25-(OH)2 vitamin D3. Physiologically, this megalin-mediated pathway is very appealing, given that the overwhelming majority of plasma 25-(OH) vitamin D3 is found in complex with DBP, with only a very small percentage of the metabolite existing in the free form(5).
In addition to the renal proximal tubules, megalin is expressed in a variety of other tissues, including ones that are steroid responsive. Notably, this endocytic receptor has been found in both male and female reproductive organs (i.e., epididymis, prostate, ovaries, and uterus) (6). Given the tissue distribution of megalin and its proposed role in cellular uptake of vitamin D, Hammes et al. (7)hypothesized that this endocytic receptor may also be important for the cellular delivery of sex steroids. This group has recently presented convincing evidence that androgens and estrogens in complex with their carrier protein, the sex hormone binding globulin (SHBG), are endocytosed in cultured cells expressing megalin. The presence of accessible megalin appears to be necessary for optimal internalization of these steroid-SHBG complexes, as evidenced by the reduced levels of uptake observed when receptor-associated protein (RAP, an antagonist of ligand binding to megalin) or megalin-specific antiserum was added, or in cells that lack megalin expression. Furthermore, transcriptional activation (the hallmark of intracellular steroid action) of an androgen-sensitive reporter was observed when androgen-SHBG complexes were added to megalin-expressing cells in vitro; this activity was also inhibited by the addition of RAP. Finally, the most striking evidence supporting a role for megalin in proper sex steroid uptake and signaling came from in vivo studies of megalin KO mice. Males lacking megalin showed impaired descent of the testes whereas megalin-deficient females exhibited abnormalities in vaginal development––both defects being consistent with insensitivity to androgens and estrogens, respectively. Hence, as is the case for megalin involvement with vitamin D3, there seems to be significant physiological relevance for megalin in the cellular uptake of sex steroids. Depending on the steroid, the majority of metabolites of certain steroids can be found in complex with their corresponding binding proteins (8). Thus, free diffusion of these molecules may not play as important a role in delivery, especially in tissues that require large amounts of steroid hormones.
Despite the intriguing findings regarding the proposed role of megalin in the endocytosis of steroid hormones, it is certainly worth noting that megalin-null mice are not phenotypic replicas of mice that lack the androgen or estrogen receptor (9). These observations suggest that megalin-independent mechanisms must also exist for the sex steroids to carry out their effects. Thyroid hormone (another small, lipophilic molecule) was also traditionally believed to enter target cells by passive diffusion; however, thyroid hormone can be actively transported into target cells via the monocarboxylate transporter MCT8 (10). It is not unreasonable to think that a similar mode of transport may occur for the sex steroid hormones.
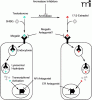
The identification of endocytic pathways for estrogens and androgens could have potentially important therapeutic implications, given the existence of breast and prostate tumors that are dependent on their respective sex steroids for growth and proliferation. If megalin is indeed the primary mediator for the uptake of these steroid hormones in pathological settings, drugs that target this endocytic receptor could help to block the supply of these molecules to cancer cells, and serve as an alternative or cooperative treatment to currently existing therapies (e.g., estrogen antagonists and biosynthesis inhibitors in the case of breast cancer) (Figure 1). There are many questions, however, that must first be addressed regarding the role of megalin in tumor biology. For example, do tumor cells that are androgen- or estrogen-dependent overexpress megalin at the plasma membrane, relative to their normal counterparts? What is the precise megalin binding site for the steroid-SHBG complex and is this site distinct for androgens versus estrogens? Given that megalin acts as a promiscuous receptor for a wide variety of ligands (e.g., vitamin and steroid–binding protein complexes, lipoproteins, enzymes, drugs, toxins, etc.), how is specificity of endocytosis achieved? More importantly, from a drug development standpoint, how might antagonism and target tissue specificity be achieved such that uptake of other endogenous megalin ligands (e.g. vitamin D3 in the kidneys) and normal physiological processes are minimally affected? Although megalin may be a promising new target for cancer therapy, much target validation work remains to be done.
In conclusion, the findings presented by Hammes et al. (7) demonstrate that megalin has a potentially significant role in sexual and reproductive development in mice. This active transport mechanism is particularly interesting, and perhaps important, regarding: 1) the proper function of tissues that require large amounts of steroids or, 2) the pathophysiological processes involved in steroid-dependent tumors. In these two examples, passive diffusion alone would be unlikely to deliver the necessary amounts of steroids to propagate their effects in target cells. Thus, the identification of megalin as an endocytic receptor for the cellular uptake of sex steroids represents a novel paradigm for a physiological role of these carrier bound molecules and should be factored into future steroid hormone biology research.

Figure 1. Schematic representation of mechanisms blocking androgen and estrogen actions. Most of the current drugs used to treat steroid-dependent tumors either: 1) block the biosynthesis of steroid hormones (aromatase inhibitors) or 2) prevent transcriptional activation of steroid responsive genes by inhibiting the binding of endogenous steroids to their intracellular receptors (androgen receptor (AR) or estrogen receptor (ER) antagonists). Megalin has been identified as an endocytic receptor for the cellular uptake of steroid/sex hormone binding globulin (SHBG) complexes (7). Development of megalin antagonists, that block the entry of steroids into cells, may serve as an alternative or synergistic treatment to presently available therapeutics.
References
1. Mendel, C.M. The free hormone hypothesis: a physiologically based mathematical model. Endocr. Rev. 10, 232–274 (1989).
2. Christensen, E.I. and Birn, H. Megalin and cubulin: Multifunctional endocytic receptors. Nat. Rev. Mol. Cell Biol. 3, 258–268 (2002). This review provides a comprehensive overview of megalin biology.
3. Willnow, T.E., Nykjaer, A., and Herz, J. Lipoprotein receptors: New roles for ancient proteins. Nat. Cell Biol. 1, E157–E162 (1999).
4. Nykjaer, A., Dragun, D., Walther, D., Vorum, H., Jacobsen, C., Herz, J., Melsen, F., Christensen, E.I., and Willnow, T.E. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3.Cell 96, 507–515 (1999). These findings provide the first suggestion that megalin is an endocytic receptor for steroid [i.e., 25-(OH) vitamin D3] delivery into cells.
5. Bikle, D.D., Gee, E., Halloran, B., Kowalski, M.A., Ryzen, E., and Haddad, J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D binding protein. J. Clin. Endocrinol. Metab. 63, 954–959 (1986).
6. Zheng, G., Bachinsky, D.R., Stamenkovic, I., Strickland, D.K., Brown, D., Andres, G., and McCluskey, R.T. Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, gp330 and LRP/alpha-2MR, and the receptor-associated protein (RAP). J. Histochem. Cytochem.42, 531–542 (1994).
7. Hammes, A., Andreassen, T.K., Spoelgen, R. et al. Role of endocytosis in cellular uptake of sex steroids. Cell 122, 751–762 (2005). This paper describes the endocytic uptake of sex steroid/carrier protein complexes by megalin, as described in this Viewpoint.
8. Dunn, J.F., Nisula, B.C., and Rodbard, D. Transport of steroid hormones: Binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J. Clin. Endocrinol. Metab. 53, 58–68 (1981).
9. Couse, J.F. and Korach, K.S. Exploring the role of sex steroids through studies of receptor deficient mice. J. Mol. Med. 76, 497–511 (1998).
10. Friesema, E.C.H., Ganguly, S., Abdalla, A., Manning Fox, J.E., Halestrap, A.P., and Visser, T.J. Identification of monocarboxylate transporter 8 as a thyroid hormone transporter. J. Biol. Chem. 278, 40128–40135 (2003).