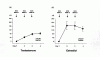Reversal of Hypogonadotropic Hypogonadism
“Therefore, brief discontinuation of hormonal therapy to assess reversibility of hypogonadotropic hypogonadism is reasonable.”
“Furthermore, the postreversal neuroendocrine profiles demonstrated activation of the hypothalamo–pituitary–gonadal axis in adulthood.”
Androgen Induced Hypogonadism (AIH) is a form of functional hypogonadism. Constitutional delay of puberty, idiopathic hypogonadotropic hypogonadism, and functional hypogonadotropic hypogonadism share several pathophysiological similarities: male predominance, familial predisposition, and disordered gonadotropin secretion.
In patients with these disorders, the response to appropriate GnRH stimuli is the restoration of secretion of luteinizing hormone and follicle-stimulating hormone, which points to the primacy of the hypothalamic disorder in the pathogenesis of the disorders The GnRH, SERM, and AI challenges are similar and share mechanisms of action.
Some patients with idiopathic hypogonadotropic hypogonadism may undergo delayed activation of the hypothalamic–pituitary–testicular axis. This finding raises the possibility that idiopathic hypogonadotropic hypogonadism, constitutional delay, and functional hypogonadotropic hypogonadism — all characterized by disordered timing or regulation of the GnRH pulse generator — may result from analogous or overlapping pathophysiological mechanisms.
In this study, 10% of the 50 men with idiopathic hypogonadotropic hypogonadism who discontinued reproductive hormonal therapy maintained adult levels of serum testosterone, revealing a relatively high incidence of reversal. There are practical implications of this observation.
They suggest that patients with idiopathic hypogonadotropic hypogonadism, with or without anosmia and regardless of their previous pubertal development, should be informed of the possibility of fertility and the spontaneous reversal of hypogonadism. In addition, men with idiopathic/functional hypogonadotropic hypogonadism should be reassessed for recovery of the hypothalamo–pituitary–gonadal axis.
The number of neurons producing GnRH in the human hypothalamus is relatively small (<2000), and these neurons are distributed as a diffuse network, rather than as a discrete nucleus. Such anatomy may render the GnRH pulse generator functionally vulnerable to minor perturbations, leading to GnRH deficiency and hypogonadotropic hypogonadism.
Although the precise mechanism of reversal of hypogonadotropic hypogonadism is unclear, the mechanism may involve plasticity of the GnRH-producing neurons in adulthood. Plasticity, defined as the ability of the nervous system to adapt in response to the environment, is a striking feature of the vertebrate brain.
Although neurogenesis in humans is thought to occur primarily during embryonic and early postnatal stages, multipotential progenitor cells residing in the subcortical white matter of the adult human brain have recently been identified as having the potential to replace neuronal lineages. Furthermore, neurons in the olfactory epithelium, the olfactory bulbs, and the dentate gyrus of the hippocampus are generated throughout life, and their generation appears to be modulated by sex steroids.
Indeed,
exposure to sex steroids, although the length of exposure was variable, seems to be a common denominator in the patients who underwent reversal. They therefore speculate that sex steroids enhance the plasticity of the neuronal network producing GnRH in the adult human brain, leading to reversal of hypogonadotropic hypogonadism.
Raivio T, Falardeau J, Dwyer A, et al. Reversal of Idiopathic Hypogonadotropic Hypogonadism. N Engl J Med 2007;357(9):863-73. MMS: Error
Background: Idiopathic hypogonadotropic hypogonadism, which may be associated with anosmia (the Kallmann syndrome) or with a normal sense of smell, is a treatable form of male infertility caused by a congenital defect in the secretion or action of gonadotropin-releasing hormone (GnRH). Patients have absent or incomplete sexual maturation by the age of 18. Idiopathic hypogonadotropic hypogonadism was previously thought to require lifelong therapy. We describe 15 men in whom reversal of idiopathic hypogonadotropic hypogonadism was sustained after discontinuation of hormonal therapy.
Methods: We defined the sustained reversal of idiopathic hypogonadotropic hypogonadism as the presence of normal adult testosterone levels after hormonal therapy was discontinued.
Results: Ten sustained reversals were identified retrospectively. Five sustained reversals were identified prospectively among 50 men with idiopathic hypogonadotropic hypogonadism after a mean ({+/-}SD) duration of treatment interruption of 6{+/-}3 weeks. Of the 15 men who had a sustained reversal, 4 had anosmia. At initial evaluation, 6 men had absent puberty, 9 had partial puberty, and all had abnormal secretion of GnRH-induced luteinizing hormone. All 15 men had received previous hormonal therapy to induce virilization, fertility, or both. Among those whose hypogonadism was reversed, the mean serum level of endogenous testosterone increased from 55{+/-}29 ng per deciliter (1.9{+/-}1.0 nmol per liter) to 386{+/-}91 ng per deciliter (13.4{+/-}3.2 nmol per liter, P<0.001), the luteinizing hormone level increased from 2.7{+/-}2.0 to 8.5{+/-}4.6 IU per liter (P<0.001), the level of follicle-stimulating hormone increased from 2.5{+/-}1.7 to 9.5{+/-}12.2 IU per liter (P<0.01), and testicular volume increased from 8{+/-}5 to 16{+/-}7 ml (P<0.001). Pulsatile luteinizing hormone secretion and spermatogenesis were documented.
Conclusions: Sustained reversal of normosmic idiopathic hypogonadotropic hypogonadism and the Kallmann syndrome was noted after discontinuation of treatment in about 10% of patients with either absent or partial puberty. Therefore, brief discontinuation of hormonal therapy to assess reversibility of hypogonadotropic hypogonadism is reasonable.
Bhasin S. Experiments of Nature -- A Glimpse into the Mysteries of the Pubertal Clock. N Engl J Med 2007;357(9):929-32. MMS: Error