Memories Can’t Wait
Researchers rethink the role of amyloid in causing Alzheimer’s
http://www.sciencenews.org/view/feature/id/70236/title/Memories_Can%E2%80%99t_Wait
By Laura Sanders
March 12th, 2011
The polite term for what Alzheimer’s disease does to the brain is “neurodegeneration.”
In reality, it’s more like violent, indiscriminate devastation. Alzheimer’s scrambles communication channels, incites massive inflammation and demolishes entire brain regions as once plump cells shrivel and die, burying memories in the wreckage. As the attack intensifies, Alzheimer’s gradually strips away a person’s mind, and ultimately the cognitive abilities that permit a conversation with a loved one, a smile or a taste of food.
A couple of decades ago, some researchers thought they knew the root cause of this brain invasion?—?dangerous buildups of a protein called amyloid-beta. Get rid of these big, sticky globs and cure the disease, the reasoning went. But in recent years, a deeper understanding of the disease, along with a few disappointing clinical trials, has challenged long-held assumptions and forced a reevaluation of this strategy.
Many researchers are convinced that A-beta is still a key target. A litany of damning evidence from genetics, pathology reports and lab experiments makes that case. Yet recent results show that A-beta is not the same foe it was originally thought to be. Smaller pieces of A-beta?—?not the large plaques that were formerly indicted?—?are likely to be malicious, capable of destroying nerve cell connections, several new studies show. Other data coming from sophisticated imaging techniques may illuminate how, when and where A-beta accumulates in the brain, and how this buildup might relate to diminished mental powers.
Yet the fact that A-beta can also accumulate in healthy brains, among other findings, has caused some Alzheimer’s researchers to shift their sights away from that protein. A new model proposes that inflammation, along with the harmful marinade it brings, might be a central cause of the disease. Other studies are turning up links between Alzheimer’s and the curious tendency of brain cells under stress to double their genetic material.
While the cause of Alzheimer’s remains elusive, the extent of its threat to the brain is becoming increasingly clear. Each week, new studies chronicle the damage in ever more detail: Chemicals that carry messages between nerve cells go MIA, brain cells’ birthrates plummet, cells’ energy output goes haywire, cell waste begins to pile up and harmful reactive chemicals get produced. Ultimately, brain cells die.
Teasing apart this tangled web?—?in which it’s nearly impossible to distinguish a diabolical mastermind from a lowly hired gun or even an innocent bystander?—?isn’t easy. If it were, the problem would be solved by now. “I think we have to be honest and say this is an incredibly complicated condition, and it’s going to be very hard to tackle it,” says Alzheimer’s researcher Lennart Mucke of the University of California, San Francisco.
A tangled web
It’s no surprise that A-beta has attracted so much attention from those intent on unraveling the mysteries of Alzheimer’s. Ominous deposits of the protein (along with tangles of another protein, called tau, that has also garnered a fair share of investigation) were what caught the eye of German physician Alois Alzheimer when he first described the disease a little over a century ago. His postmortem exam of a patient’s brain revealed the amyloid plaques that have been associated with Alzheimer’s disease ever since.
But much remains unknown about A-beta. While it has been shown that A-beta is a snippet cut from the larger amyloid precursor protein, found in nearly every cell in perfectly healthy brains, A-beta’s normal function remains murky. Studies have hinted that the protein might aid nerve cell activity or combat dangerous pathogens. Others suggest A-beta is merely a cellular by-product that adopted a new and damaging role.
In the tangle of Alzheimer’s, one thing is clear: Old age is the No. 1 risk factor, a frightening realization as the front edge of the baby boomer tide turns 65 this year. The disease is “obviously an epidemic of staggering proportions, and obviously of great economic impact,” says neuroscientist Sam Sisodia of the University of Chicago. Alzheimer’s is the fastest growing cause of death from major disorders in the United States, and a recent analysis estimates that the nation’s annual cost of Alzheimer’s-related care will exceed $1 trillion by 2050.
Alzheimer’s is unlike anything else clinicians have treated: In most cases, no one knows what causes it. It can’t be definitively diagnosed until a pathologist cuts into the dead brain. There is no known cure or therapy for prevention, and even if there were, it wouldn’t be clear when to use either one. Many believe the disease causes its irreparable damage years before symptoms appear.
“We have therapies that help with the symptoms, but we don’t have disease-modifying treatments,” says Paul Aisen of the University of California, San Diego School of Medicine, a neurologist who tests potential Alzheimer’s drugs. “And we don’t know what the best target is, and we don’t know what the best timing is.”
But many scientists in the field find hope in the fact that they have sketched out the broad outline of how the disease works, pointing to new targets for therapies. One key to filling in that sketch, scientists now know, is understanding brain cell communication.
Brain chatter, interrupted
A-beta scrambles neural dispatches in an unexpected way, new work from neuro¬scientist Gabriel Silva of the University of California, San Diego suggests. In a dish of brain cells called astrocytes, a droplet of the A-beta protein sparked a signal that can silence chatter between nerve cells, the brain’s main communicators. The signal traveled as a wave of calcium atoms that washed across cells, kicking off a series of damaging events that could end with disrupted nerve cell communication.
“Amyloid-beta is sufficient, completely on its own, to induce these things,” Silva says of the finding, which was published last year in ASN Neuro. These calcium waves have also been spotted in mice loaded down with the human form of A-beta to mimic the high levels found in some Alzheimer’s patients. (A-beta doesn’t usually accumulate in the brains of mice.) It’s still not clear whether A-beta triggers calcium waves in human brains.
A-beta probably has a more direct path to harming synapses, the junctures where messages, including those that create memories, are transmitted between nerve cells. In mice, an abundance of A-beta can order an assassination of a protein that’s important for forming memories, a study by Mucke and colleagues published in the Jan. 6 Natureshowed.
Normally this protein, called EphB2, oversees the action of a signaling molecule that moves across synapses and helps create new memories. In the experiments, A-beta latched on to EphB2 and helped move it to the cellular dump. Without the right levels of EphB2, synapse-traveling molecules went haywire. “Nerve circuits couldn’t perform properly anymore, and the mouse couldn’t learn or remember properly,” Mucke says. “The whole information processing pathway comes apart.”
Mucke and his colleagues reversed these memory deficits in mice carrying heavy loads of A-beta by boosting levels of the EphB2 protein. Studies show that people with Alzheimer’s have less EphB2 in their brain cells, so protecting the protein from A-beta or artificially boosting its levels might be a way to reverse cognitive decline, Mucke says.
A-beta may also hit another target at the synapse. In mouse brains with high levels of A-beta, a protein called Caspase-3 was busier than normal, a dangerous hyperactivity that led to the disintegration of dendrites, a nerve cell’s message-receiving extensions. This A-beta–Caspase-3 combo caused dendrites’ demise in the hippocampus, the brain’s center for forming memories, researchers reported in the January issue of Nature Neuroscience. Dampening Caspase-3’s activity protected these dendrites, suggesting that, like EphB2, Caspase-3 might be a good place to intervene to protect nerve cell communication from Alzheimer’s disease.
Small but dangerous
These assaults at the synapse were led by diminutive forms of A-beta called oligomers. Small, dissolvable pieces of A-beta, they are the building blocks of the large, insoluble fibrils that form the plaques first spotted by Alois Alzheimer. Oligomers are quickly gaining notoriety as a more probable villain than the well-studied plaques.
Data from neuroscientist Caleb Finch’s group at the University of Southern California in Los Angeles, and work by other researchers, have made the case that the oligomers are the most damaging form of A-beta. “We are convinced that the oligomeric forms, small assemblies of three to 10, are more toxic than the long fibrils,” Finch says.
In fact, mice with a form of A-beta that can’t accumulate into large fibrils still show memory troubles, Takami Tomiyama of Osaka City University Graduate School of Medicine in Japan and colleagues reported last year in the Journal of Neuroscience.
This result “adds powerfully to our theory,” Finch says.
Right now, there’s no way to visualize these A-beta oligomers in a living human brain. Autopsies and recent developments in brain imaging allow researchers to see larger A-beta plaques, but working backward from the plaque to estimate amounts of the smaller oligomers is tricky. This elusive relationship, says Sam Gandy of Mount Sinai Medical Center in New York City, throws a wrench in studying oligomers in the brain. “It’s really hard to get a good accounting of how much is there.”
Lots of simulations and test-tube experiments have attempted to demystify the oligomer-plaque relationship?—?for example, by considering whether there’s a critical mass of oligomers required for plaque formation. But the “exploded drugstore” in the brain confounds the math, Finch says. Chemicals and salts floating around in the brain may influence the conversion rate of A-beta oligomers into plaques. “You can do beautiful model assemblies in a test tube … but how relevant that is to the mess of small molecules in the brain is imponderable,” he says.
A-beta logistics
Even though it’s not yet clear how to measure oligomer levels from plaque, or vice versa, new brain imaging techniques may help clear up another problem: identifying who’s at risk.
In 2002, University of Pittsburgh researchers William Klunk and Chester Mathis tested a compound, Pittsburgh Compound B or PiB, that sticks to plaques of A-beta in the brain and may serve as an Alzheimer’s beacon in an imaging scan. Though relatively new, PiB is gaining more and more credence as a reliable measure of A-beta plaques. An autopsy on the first Alzheimer’s patient to ever undergo a PiB scan confirmed that the tracker was indeed detecting A-beta plaques, Swedish researchers reported January 1 inBrain.
A major question researchers expect PiB to help answer is when A-beta buildup starts. Though PiB hasn’t been around long enough for long-term studies, preliminary results suggest that A-beta plaques appear years before brainpower declines. Healthy people with a strong PiB signal in their brains are more likely to exhibit mild dementia within the next few years, a small study published in 2009 in the Archives of Neurology found.
This potentially long lag time between the start of the disease and debilitating symptoms fits with clinical observations, says neurologist Randall Bateman of Washington University School of Medicine in St. Louis. “Clinical symptoms are only seen when the neurons are dead,” he says. “We know that people aren’t symptomatic until they lose 60 to 70 percent of the neurons in key brain regions.”
Waiting until a person exhibits severe cognitive problems and then trying to reverse them is like “throwing a rope to a guy that’s already jumped off the building,” says neuroscientist Charles Glabe of the University of California, Irvine, who is working on a vaccine-based strategy to decrease A-beta in the brain. “He’s going to hit.”
Modern medicine’s approach to treating heart disease isn’t to withhold therapies until after the heart fails, Aisen and colleagues pointed out January 18 in Neurology. Once treatments are found, figuring out exactly when Alzheimer’s sets in will probably help to make them much more effective.
Yet caution is needed when interpreting a PiB-positive or PiB-negative brain scan, especially when considering estimates that 20 to 50 percent of healthy people go about their business with brains chock-full of A-beta plaques. Many of those fully functional brains would easily earn an Alzheimer’s diagnosis with PiB scanning. It’s not clear whether, if people were to live long enough, anyone walking around with A-beta plaques in the brain would eventually succumb to Alzheimer’s.
“If I’m cognitively normal, do I care if I have amyloid in my brain?” Klunk said at the 2010 Society for Neuroscience meeting in San Diego. “Is it irrelevant, or is it like blood pressure, where you’re not sick but you’re walking around with 200 over 120? That’s not a good thing.”
A-beta buildup may be the most obvious, common and even leading cause of Alzheimer’s. But for some people, A-beta may indeed be irrelevant.
A new battle plan
Neurobiologist Karl Herrup of Rutgers University says that the idea of Alzheimer’s without A-beta must be considered. Herrup points to patients who exhibit all of the cognitive impairments that follow Alzheimer’s disease, yet for whom subsequent imaging experiments or postmortem tests find no plaques in the brain. “When I talk to clinicians about it, they all agree that this is a real category, that it’s not just the occasional person.”
Other pieces of evidence don’t add up either, Herrup says. The presence of A-beta plaques in cognitively healthy people raises doubts about A-beta as the bad actor it was once assumed to be. So does A-beta’s failure, in mice, to elicit the kind of massive and widespread neuron death seen in Alzheimer’s.
“We’ve filled mouse heads with plaques, and oligomers for that matter,” Herrup says. “And what we’ve created is, at best, mild cognitive impairment…. If you go to the Alzheimer’s ward of any institution, most of the residents there would be ecstatic to be returned to the level of function in our worst mouse model.”
Another particularly troubling piece of data is that, so far, lowering A-beta levels in human brains hasn’t improved brainpower. A drug called bapineuzumab, thought to shuttle A-beta out of the brain, decreased amyloid plaques in the brain but didn’t boost brainpower in patients with mild to moderate disease, a clinical trial published in Lancet Neurology last year showed.
“The main issue is that none of these amyloid-lowering therapies have improved cognitive function,” Gandy says. “If there had been a benefit to any of the things that lowered amyloid, then that would obviously put all the doubt to rest.”
That’s not to say A-beta doesn’t have a role in the disease. But in some cases A-beta may not be leading the charge.
Instead, Herrup proposes a new model of how Alzheimer’s disease sets in and spreads?—?a model that moves A-beta out of the limelight. First comes an injury, which may be related to some sort of vascular event such as a microstroke or mild head trauma suffered during a fall. This minor event then kicks off an inflammatory response in the brain.
This inflammation, Herrup and others argue, may be at the core of Alzheimer’s disease. The “inflammation hypothesis” holds that given enough time, the harmful stew of factors triggered by an injury can cause major, irreversible damage to brain cells.
Herrup’s model demotes A-beta but doesn’t discharge it entirely. It turns out that A-beta probably worsens inflammation, and inflammation may spur more A-beta formation.
“The major issue is chicken and egg,” says Finch. “Right now, I don’t think the cause-and-effect aspect of inflammation in Alzheimer’s disease can be resolved, but everybody recognizes that it’s enmeshed in the process in a fundamental way.”
Extended inflammation triggers a permanent change in brain cells, a point of no return, Herrup proposes. “The cells don’t care any longer about whether there’s amyloid in their environment, or whether there’s inflammation in their environment,” he says. “They have crossed the Rubicon.” After this point, no intervention or therapy could help.
Herrup’s hunch is that this switch might be related to a curious fact about neurons: When they’re under stress, they duplicate their genetic material. Usually, when most cells in the body do this, it’s in preparation for replication of the whole cell, and the new copy of the cell gets the extra set of DNA. But instead of dividing, neurons under duress just chug along with double the amount of DNA. Once the DNA is doubled, there’s no way for a brain cell to get rid of it, short of dividing. And neurons don’t divide.
“I’m afraid it’s been one of those observations that no one can fit anywhere, so they always smile and say, ‘Well, that’s really interesting,’ and go back to what they were doing,” Herrup says.
Researchers don’t yet know whether this extra DNA is harmful, but it’s clear that the stunted duplication occurs more in brain cells battling Alzheimer’s. In people with the disease, not only were brain cells more likely to have extra copies of DNA, but those cells were also at a greater risk of death, German researchers suggested in a paper published in July in the American Journal of Pathology.
The link between extra DNA and neurons fated for destruction, though intriguing, is preliminary. It may turn out to be another red herring on the quest to find Alzheimer’s ultimate cause. Neatly assigning roles to the entire cast of characters at work in Alzheimer’s disease, and finding ways to counteract them, remains challenging.
“How close are we to understanding Alzheimer’s disease? That’s the same question we were asking 10 years ago,” Sisodia says. “And we’ll be asking that same question 10 years from now.”
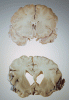
Differences in a healthy brain (top) and a diseased one (bottom) clearly show the damage wrought by Alzheimer’s.
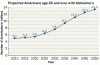
As the baby boomers age, the number of elderly Americans with Alzheimer’s is projected to reach 13.5 million by 2050. Assuming no breakthroughs in treatment, health care costs will continue to increase as well.
Though amyloid-beta has long been implicated in Alzheimer’s disease, figuring out just how the protein harms the brain has proved tricky. A-beta appears to cause problems when it aggregates into other forms and accumulates, perhaps because of overproduction or slow clearance. In its small oligomer form, A-beta can damage synapses, message-relaying connections between nerve cells. By setting off cascades of reactions, oligomers can also damage neural extensions called dendrites. Oligomers can clump into fibrils, which then form the large amyloid plaques characteristic of the disease. Many other potential culprits are also being investigated, some of which alter A-beta activity and some of which appear to cause damage in different ways.

In a PET scan, labeled PiB protein lights up when bound to amyloid plaques in the brain. A healthy person (top) has little binding compared with the high levels seen in an Alzheimer’s patient (middle). But high levels are also found in some healthy people with no cognitive problems (bottom).
A small number of Alzheimer’s interventions, some highlighted below, are currently in Phase III clinical trials, which are undertaken after preliminary studies show that a drug is safe and may be effective. Right now, more than 800 clinical trials?—?including those at earlier stages of testing?—?are searching for potential Alzheimer’s therapies. Among those are therapies that examine the effects of exercise, diet and inflammation-reducing drugs.