Type-IIx
Member
Clenbuterol focus: Research on Clen & Beta2- Adrenergic Compounds, Protocols
Author: Type-IIx
Research considerations
Clenbuterol is a drug legally used in human medicine in a select few countries as a bronchodilator at doses up to 40µg daily [18]. Primarily, it is used illicitly as a potent repartitioning agent to promote growth in cattle and sheep by increasing protein accretion and fat removal with little or no change in body weight, and by human athletes for this effect [8].
This work thus makes some extrapolation from animal research on clenbuterol as well as from human research on similar compounds (i.e., salbutamol/albuterol, terbutaline).
Pharmacokinetics
Clenbuterol is a β₂-adrenergic agonist similar in some structural respects to salbutamol (albuterol). Agonism of the β₂ receptor stimulates adenylyl cyclase activity which ultimately leads to downstream effects of smooth muscle relaxation in the bronchioles as a therapeutic target. Clenbuterol is a potent sympathomimetic.
Oral bioavailability of 70-80% and a long half-life of 25-39 hours [6].
Desensitization
Tachyphylaxis, or desensitization, is a feature of the β₂AR. This is likely because β₂AR activation and stimulation of downstream effects are a target for phosphorylation (50) and/or because it binds β-arrestin (51), an accessory protein involved in G protein-coupled receptor desensitization (52) [16].
Tolerability
High doses of clenbuterol have been used in the literature. The highest doses used are for patients with LVAD (left ventricular atrioventricular devices) and congestive heart failure. For this purpose, clenbuterol is used specifically for its effects on cardiac remodeling! It is important to note that cardiac remodeling should generally be avoided by healthy persons (though the remodeling from clen is ostensibly not maladaptive)! There was no change in LV mass with 80 µg daily for 3 months, [19].
Otherwise, an important research consideration is that tolerability cannot be assessed nor inferred from dosages administered in studies. Rather, tolerability must be assessed as an endpoint in the trial, in order to be able to make a claim regarding the drug's tolerability.
Here, this author presents some actual data on tolerability where it has been assessed as an end-point.
From a study on patients with heart failure. Here, the dosage started at 20µg twice daily and was titrated up to 40µg twice daily after a week [19]. Two of the nineteen (2/19) subjects dropped out of the 3 months-long study due to clenbuterol side effects:
Two clenbuterol subjects required discontinuation of study drug (asymptomatic slow ventricular tachycardia, severe muscle cramps without significant elevation increatine kinase [CK]) [19].
One further clenbuterol subject had a high rate of ventricular ectopy that disappeared without reduction in the clenbuterol dose [19].
One placebo subject had frequent non-sustained ventricular tachycardia. There were no implantable cardioverter-defibrillator discharges during the course of the study [19].
Six clenbuterol and 2 placebo subjects reported mild muscle cramps [19].
The CK value was elevated in 5 clenbuterol and 4 placebo subjects. The range of peak CK was 300 to 597 mg/dl in clenbuterol subjects and 305 to 408 mg/dl with placebo. Three clenbuterol subjects had cramps without elevation of CK, and CK was elevated in 1 clenbuterol subject who was asymptomatic. Of importance was that the CK level decreased despite continued drug administration (Figure 1B) [19].
Tremors were reported in 5 clenbuterol and 2 placebo subjects [19].
In a large, double-blind clinical trial (n=175 women with stress incontinence, 82 received 40µg clen daily for 2 weeks):
Side effects were noted in 13 clenbuterol-administered patients (15.9%), and the treatment was discontinued in 5 of these (due to tremors of the finger in 2 cases; dizziness in 1; urinary hesitancy in 1; and loss of appetite in 1). In the remaining 8 patients the side effects were mild and the treatment was able to be completed. In the clenbuterol group, the main side effect was finger tremors (8 out of 82, 9.8%), and tachycardia was also noted (2 out of 82, 2.4%) [20].
In the placebo group, side effects were noted in 12 patients (12.9%), and the treatment was discontinued in 4 of these (dizziness in two patients and nausea in two. One of these 4 patients was treated for longer than 1 week, and the data were thus included in the statistical analysis). In the placebo group, the main side effects were gastrointestinal disturbance (7 out of 93, 7.5%) and dizziness (3 out of 93, 3.2%) [20].
Side effects
- Electrolyte disturbance (primarily hypokalemia and hyperglycemia) and associated muscle cramping
- Tachycardia
- Dyskinesia
- Tremor
- Liver failure
- Muscle atrophy
- Myocardial infarction
- Myocardial reperfusion injury
Cardiac effects
The infamous rat study
"myotoxic" cardiac necrosis
Provides evidence of cardiotoxicity with high doses in rats:
Burrinston et al. (21) explored the myotoxic effects of clenbuterol injected subcutaneously. Rats were treated with a single shot of clenbuterol. The amount of necrosis in the muscle was measured using an anti-myosin monoclonal antibody that enters the endoplasmic reticulum only in cells that have undergone necrosis. The amount of necrosis in the heart was positively correlated with the amount of clenbuterol that was injected. Necrosis was seen at 4 hours post administration and peaked at 15 hours. This finding can explain the cardiomyocyte damage seen after a single ingestion of clenbuterol. Necrosis decreased by 91-100% in rats pre-treated with bisoprolol, β2 blockade or noradrenaline depletion. This finding is consistent with direct myotoxic effect of clenbuterol. Peak cardiomyocyte necrosis was noted 2.4 mm from the apex. This finding is consistent with the high incidence of ECG and echocardiographic changes in the in the inferior and lateral wall in the patients presented. Direct myocardial injury due to catecholamine activation has been well described and attributed to supply/demand imbalance induced by the sustained activation of adrenergic receptors and mitochondrial dysfunction (22) [6].
These pathological changes are not to be taken lightly. Clen causes real cardiac harm at performance- and physique- enhancement dosages.
Interestingly,
Case reports of real myocardial injury
See [6].
A common theme of absurdly high doses.
Mechanisms of myocardial injury
cardiac necrosis
[Clenbuterol-induced myocyte necrosis] appear to be driven through clenbuterol-induced pre-synaptic release of catecholamines within the myocardium which cause myocyte damage through a β1-specific mechanism. Such effects can be blocked in vivo by the use of β1-specific antagonists [e.g., bisoprolol] [9].
apoptosis = cell death
The lowest dose of clenbuterol to induce cardiomyocyte apoptosis was 1 microg/kg, with peak apoptosis (0.35 +/- 0.05%; P < 0.05) occurring in response to 5 mg/kg. In the soleus, peak apoptosis (5.8 +/- 2%; P < 0.05) was induced by the lower dose of 10 microg/kg. Cardiomyocyte apoptosis was detected throughout the ventricles, atria, and papillary muscles. However, this damage was most abundant in the left ventricular subendocardium at a point 1.6 mm, that is, approximately one-quarter of the way, from the apex toward the base. beta-AR antagonism (involving propranolol, bisoprolol, or ICI 118551) or reserpine was used to show that clenbuterol-induced myocardial apoptosis was mediated through neuromodulation of the sympathetic system and the cardiomyocyte beta1-AR...
[15]
cardiac hypertrophy
In rat cardiocytes (heart cells), Clenbuterol is associated with a threefold increase in IGF-I mRNA expression. An increase in ANP, BNP, but not αSkA indicates physiological cardiac hypertrophy. Cardiac fibroblasts contain essentially only β2 receptors and are the prime targets of clenbuterol indicating a paracrine signalling role for physiological cardiac hypertrophy. Hypothesis that the β₂-AR physiological function is myocardial protection against stress [9].
? Liver effects
The results from this poor study showed a significant between-group difference (Clen+Training vs. Training) in ALT elevation, with the Clen+Training group showing a higher (significant) elevation in ALT [18]. The (apparent) cross-sectional study ("all groups followed a special diet program... rich in protein, moderate carbohydrate with low fats"... "training in gym with special training program put and designed by their coach [not described]. The "cycle" was absurd (See #Abdulredha protocol). It is not completely clear whether the study is a crossover or an interventional design given that they used statistical methods (repeated latin square design) and made a curious statement "...22 male who are training plus taking Clenbuterol (T+Clen.) (NOTE: take it by their will.)" The authors of this paper fail to consider whether their small group sample for Clen+Training contains an AAS user (which would actually be expected to impact these liver values and lipids. The repeated latin square design carries a risk of the carry-over effect, which certainly applies with training for all variables measured.
Skeletal muscle hypertrophy ↑
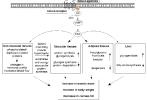
cAMP signaling and mechanisms of action downstream of clen's β2AR agonism
[3]
Mechanisms of skeletal muscle hypertrophy
--------------------------------------------
β2-agonists
Epinephrine interacts with the β2 adrenergic receptor (β2AR), a G protein-coupled receptor coded by the ADRB2 gene, which is the most abundant adrenergic receptor present in muscle fibers.
- β2 agonists (e.g., clenbuterol) =[binds]=> β2AR ⇒ activates adenylate cyclase ⇒ ↑cAMP, protein kinase A (PKA)
- Chronic treatment (e.g., clenbuterol) leads to hypertrophy through poorly defined pathways appearing to involve the IGF1-PI3K-Akt-mTOR cascade.
- PKA-dependent phosphorylation of the transcription factor CREB (cAMP response element binding protein) and associated
coactivators play a largely unknown role
- MEF2 (+) [pro-hypertrophic coactivator] may be involved
------------------------------------------

[10]
Abo et al. (1) observed that expression of myostatin was higher after 21 days of clenbuterol treatment with no differences in the first week of treatment, suggesting that myostatin functions as a negative regulator in the latter stages of β2-agonist treatment. Nonetheless, while the present observations suggest that follistatin may play a role in β2-adrenergic mediated hypertrophy, the mechanisms are complex and possibly involve an interplay of several factors that directly or indirectly attenuate the negative regulatory action of the myostatin system on growth (8, 54, 55, 72, 75) [12].
mTOR phosphorylation
Human skeletal muscle mTOR phosphorylation ↑121% (potent activator) [24].
Strength, sprint, power ↑
In the overall analyses on healthy non-asthmatic subjects, prohibited β2-agonists (note: clen was not the subject of any study, rather oral salbutamol (albuterol) would be a prohibited agent studied) improved anaerobic performance (0.46 above the expected mean) related to dose (e.g., prohibited) and route of administration (e.g., oral), and a tendency toward greater effect with multiple weeks of treatment [4]. This corresponded to a 70 m sprint time and MVC improvement by 5% in competitive athletes and high performance cyclists and triathletes, respectively. The % improvement was 3% in sprint and 6% in strength in the respective populations [4].
In a study of maximal cycling sprint performance:
Enhancements in muscle force and power output during 30 s of maximal cycling induced by chronic β2-adrenergic stimulation in humans primarily are explained by skeletal muscle hypertrophy. In addition, that change in amount of MHC IIa isoforms and in expression of proteins involved in lactate production (LDH), Ca²⁺ uptake (SERCAI), and oxidative phosphorylation (OXPHOS complex V) of skeletal muscle mediated by chronic β2-adrenergic stimulation were complementary mechanisms for enhancements in power output during 30 s of maximal cycling [12].
We observed that the increase in lean body mass induced by terbutaline treatment was 3% when measured by DXA-scan, whereas the increase observed in CSA of muscle fibers was 13–15% [12].
Peak power and mean power:
Clen, postoperatively, increased leg strength (knee extensor maximum voluntary isometric force) at 40µg daily (20µg 2x) for 6 weeks [17]. While the unoperated leg (consider bilateral strength connection, loss of strength for a long duration prior to study) rebounded in strength in the PLA group (+12N, 1.56%, E.S. 0.44), there was a significant increase in the EXP group of +78N, 10.26%, E.S. 2.29 for clen [17]. There were insufficient participant subjects due to equipment failure for a measure of muscle CSA to draw conclusions from (type 2 error sampling likely; computed tomography insensitive). Strength was maintained throughout the two week washout period [17] (distinguish from salbutamol [albuterol]).
Mechanisms in strength and power increase
Muscle contractile properties
Maximum voluntary contraction (MVC) and Peak Twitch Force:
Significant interactions (treatment x time) were observed for MVC (P0.01) and peak twitch force (P0.01) between TER and PLA with the intervention. TER increased MVC by 97 ± 29 N and peak twitch force by 67 ± 14 N compared with PLA. Degree of voluntary activation level, time-to-peak twitch force, and half-relaxation time did not change with the intervention in either group [12].
Body composition
↑ Lean body mass and ↓ fat mass:
We observed that the increase in lean body mass induced by terbutaline treatment was 3% when measured by DXA-scan, whereas the increase observed in CSA of muscle fibers was 13–15% [12].
Mechanisms in lipolysis
Upon activation of the β2-adrenoreceptor on adipocytes (fat cells) located in the plasma membrane:
RMR increase
RMR increase in humans:
80 μg clenbuterol ↑RMR 21% over 3 hr (78 kg bodyweight men), fat oxidation ↑39% [24].
Metabolism
↑plasma concentrations of glucose (+25%), lactate (+87%), insulin (+105%), fatty acids (+129%) [24].
Oxidative capacity
No diminished oxidative capacity in humans.
Endogenous GH effects
β-adrenergic blockade/antagonism enhances the GH response to GHRH (secreted by the hypothalamus) but has no apparent effect on spontaneous GH secretion (Muller, 1987; Guistina & Veldhuis, 1998; Martha, Blizzard & Rogol, 1988) [11].
Administration of salbutamol (albuterol) [i.e., clenbuterol], a β₂-adrenergic agonist, inhibits GH secretion and is able to block the stimulation of GH release by L-arginine or pyridostigmine (Ghigo et al., 1994) [11].
Other pharmacological agents, receptors, and putative mechanisms
Nicotinic cholinergic and α₁-adrenergic receptors appear to have lesser effects on GH secretion (Muller, 1987; Guistina & Veldhuis, 1998) [11]. Antagonists of α₂-adrenergic receptors (e.g., yohimbine) can completely block the stimulatory effects on GH secretion of enhancing cholinergic tone with pyridostigmine, a cholinesterase inhibitor (Devesa et al., 1991) [11]. Administration of the α₂-adrenergic agonist clonidine stimulates GH secretion (Miki, Ono, & Shizume, 1984) [11].
Conclusion: Most experimental evidence supports the hypothesis that activation of β-adrenergic receptors increases hypothalamic somatostatin secretion (Guistina & Veldhuis, 1998) [11].
Adjunct drugs
rhGH ✓
Since the primary purpose of clen is lipolysis/recomp it makes a great deal of sense to use rhGH for its additional and potent effects for the same goal.
rhGH exerts its lipolytic effect via multiple pathways including some regulation of the adrenergic system. Some evidence suggests increased beta1 and beta3 receptor function [13] - indicating potential synergism (albeit without a reduction in the effective dose of clen) due to effects on different mediators of lipolysis.
Further, clen as a β-adrenergic agonist inhibits endogenous GH secretion [11] indicating strongly for the administration of exogenous (rh)GH.
++ additional lipolytic/recomp effects
++ ameliorates the inhibition of endogenous GH secretion
Taurine ✓
Muscle cramps due to electrolyte imbalance (hypokalemia and hyperglycemia mostly) may be ameliorated by oral administration of Taurine.
Oral administration of taurine in healthy individuals gave a plasma elimination half-life that ranged from 0.7-1.4 h [14]. In healthy individuals a clearance rate that ranged from 14 to 34.4 L/h [14].
+ modulation of intra- and trans- cellular electrolytes in skeletal muscle
Putative mechanism
Repetitive activity would be sustained by potassium accumulation in the T-tubular system (1, 13, 14), which would in turn keep the membrane potential sufficiently depolarized for potassium conductance to be inactivated through the anomalous rectification mechanism (6). The rhythmic inactivation of potassium conductance would probably account for repetitive action potentials (6). The depressant action of taurine upon excitable cells has been largely substantiated in studies of neural, retinal, or cardiac tissues (11, 37, 39), but only scattered experiments have focused on the effects of this amino acid upon skeletal muscles (5, 18). Taurine has been shown to hyperpolarize muscle or nerve cells (18,23), and this effect can be ascribed to an increment of intracellular potassium concentration (18, 40) as well as to an increase of potassium and chloride conductances, possibly by modulation of the availability of intracellular calcium (11, 25). [23]
Bisoprolol, Metoprolol ✓
β1 specific antagonist
Bisoprolol is an antihypertensive drug routinely prescribed in medicine with specific antagonism of the β1-AR and reduction in the RAAS.
The mechanism by which clen may induce cardiac necrosis involves catecholamine activation of the β1-adrenergic receptors [9]. In order to prevent this myotoxic effect, a β1 antagonist, bisoprolol, should be used if accessible.
Though the mechanism of action of bisoprolol has not been fully elucidated in hypertension, it is thought that therapeutic effects are achieved through the antagonism of β-1adrenoceptors to result in lower cardiac output. Bisoprolol is a competitive, cardioselective β1-adrenergic antagonist. When β1-receptors (located mainly in the heart) are activated by adrenergic neurotransmitters such as epinephrine, both the blood pressure and heart rate increase, leading to greater cardiovascular work, increasing the demand for oxygen. Bisoprolol reduces cardiac workload by decreasing contractility and the need for oxygen through competitive inhibition of β1-adrenergic receptors. Bisoprolol is also thought to reduce the output of renin in the kidneys, which normally increases blood pressure. Additionally, some central nervous system effects of bisoprolol may include diminishing sympathetic nervous system output from the brain, decreasing blood pressure and heart rate. (DrugBank)
++ prevents cardiac toxicity
The evidence does not support the use of any of these following drugs in combination with clen to counter the β-adrenergic receptor downregulation:
Ketotifen ✖
- Inhibits cAMP activity (clen's primary target of action)
Yohimbine ✖
- Inhibits GH secretion
- further increases sympathetic drive
- The risk or severity of hypertension can be increased when Yohimbine is combined with Clenbuterol (DrugBank)
+ synergistic/additive lipolytic effect possible via action at different (α₂-adrenergic) receptors
Benadryl ✖
Diphenhydramine
- The risk or severity of Tachycardia can be increased when Diphenhydramine is combined with Clenbuterol. (DrugBank)
Practical
Llewellyn protocol
...Copyright...
Abdulredha protocol
This protocol comes from an actual (terrible) research study [18] from the Iraq Medical Journal (2019). Its namesake is its progenitor, Dr. Abdulredha. Do not use this protocol: it is an example of atrociously bad research.
Dr. Abdulredha proposes 6 cycles (12 weeks) of the following protocol:
Day 1: 20µg, Day 2: 40µg, Day 3: 60µg, Day 4: 80µg, Day 5: 100µg, Day 6: 120µg, Day 7: 140µg
Day 8: 140µg, Day 9: 120µg, Day 10: 100µg, Day 11: 80µg, Day 12: 60µg, Day 13: 40µg, Day 14: 20µg
[18]
Modern protocol
I have a great deal to say about my extrapolation (and implementation) of practical use from the data on clen, beta2-adrenergic agonists, and PEDs generally.
I am now offering consulting for a fee for individualized performance- and physique- enhancement protocols and answering all questions, with support for all statements made with references and explanation of logic, on any and all questions related to the science and practice of PEDs, with live chat scheduled.
If you are serious about consulting, you may contact me here via PM with the message titled “Consulting” and we can discuss.
______________________________
References:
[1] GEORGE, I., XYDAS, S., MANCINI, D., LAMANCA, J., DITULLIO, M., MARBOE, C., … PETRILLI, C. (2006). Effect of Clenbuterol on Cardiac and Skeletal Muscle Function During Left Ventricular Assist Device Support. The Journal of Heart and Lung Transplantation, 25(9), 1084–1090. doi:10.1016/j.healun.2006.06.017
[2] YAMAMOTO, I., IWATA, K., & NAKASHIMA, M. (1985). Pharmacokinetics of plasma and urine clenbuterol in man, rat, and rabbit. Journal of Pharmacobio-Dynamics, 8(5), 385–391. doi:10.1248/bpb1978.8.385
[3] Parr MK, Müller-Schöll A. Pharmacology of doping agents—mechanisms promoting muscle hypertrophy. AIMS Molecular Science 2018;5:145-55.
[4] Riiser A, Stensrud T, Stang J, Andersen LB. Can β2-agonists have an ergogenic effect on strength, sprint or power performance? Systematic review and meta-analysis of RCTs. Br J Sports Med. 2020 Nov;54(22):1351-1359. doi: 10.1136/bjsports-2019-100708. Epub 2020 Aug 3. PMID: 32747344.
[5] Schiaffino, S., Reggiani, C., Akimoto, T., & Blaauw, B. (2020). Molecular Mechanisms of Skeletal Muscle Hypertrophy. Journal of Neuromuscular Diseases, 1–15. doi:10.3233/jnd-200568
[6] Shafrir A, Leibowitz DW, Alcalai R, Elitzur Y, Muszkat M. Myocardial injury induced by the long acting beta2 adrenergic agonist clenbuterol. Cardiol Cardiovasc Med 2019;3(4):186–192.
[7] Witkowska-Piłaszewicz O, Pingwara R, Szczepaniak J, Winnicka A. The Effect of the Clenbuterol-β2-Adrenergic Receptor Agonist on the Peripheral Blood Mononuclear Cells Proliferation, Phenotype, Functions, and Reactive Oxygen Species Production in Race Horses In Vitro. Cells. 2021 Apr 17;10(4):936. doi: 10.3390/cells10040936. PMID: 33920705; PMCID: PMC8072563.
[8] SLEEPER, M. M., KEARNS, C. F., & McKEEVER, K. H. (2002). Chronic clenbuterol administration negatively alters cardiac function. Medicine & Science in Sports & Exercise, 34(4), 643–650. doi:10.1097/00005768-200204000-00013
[9] Bhavsar, P. K., Brand, N. J., Felkin, L. E., Luther, P. K., Cullen, M. E., Yacoub, M. H., & Barton, P. J. R. (2010). Clenbuterol Induces Cardiac Myocyte Hypertrophy via Paracrine Signalling and Fibroblast-derived IGF-1. Journal of Cardiovascular Translational Research, 3(6), 688–695. doi:10.1007/s12265-010-9199-1
[10] Meyer, H. H. D. (2001). Biochemistry and physiology of anabolic hormones used for improvement of meat production. APMIS, 109(1), 1–8. doi:10.1111/j.1600-0463.2001.tb00010.x
[11] Growth Hormone in Adults: Physiological and Clinical Aspects, Second Edition. 2000.
[12] Hostrup, M., Kalsen, A., Onslev, J., Jessen, S., Haase, C., Habib, S., … Bangsbo, J. (2015). Mechanisms underlying enhancements in muscle force and power output during maximal cycle ergometer exercise induced by chronic β2-adrenergic stimulation in men. Journal of Applied Physiology, 119(5), 475–486. doi:10.1152/japplphysiol.00319.2015
[13] Yang S, Mulder H, Holm C, Edén S. Effects of growth hormone on the function of beta-adrenoceptor subtypes in rat adipocytes. Obes Res. 2004 Feb;12(2):330-9. doi: 10.1038/oby.2004.41. PMID: 14981226.
[14] Ghandforoush-Sattari M, Mashayekhi S, Krishna CV, Thompson JP, Routledge PA: Pharmacokinetics of oral taurine in healthy volunteers. J Amino Acids. 2010;2010:346237. doi: 10.4061/2010/346237. Epub 2010 Jun 29.
[15] Burniston JG, Tan LB, Goldspink DF. beta2-Adrenergic receptor stimulation in vivo induces apoptosis in the rat heart and soleus muscle. J Appl Physiol (1985). 2005 Apr;98(4):1379-86. doi: 10.1152/japplphysiol.00642.2004. Epub 2004 Dec 10. PMID: 15591297.
[16] Collins, S., Cao, W., Daniel, K. W., Dixon, T. M., Medvedev, A. V., Onuma, H., & Surwit, R. (2001). Adrenoceptors, Uncoupling Proteins, and Energy Expenditure. Experimental Biology and Medicine, 226(11), 982–990. doi:10.1177/153537020122601104
[17] Maltin, C. A., Delday, M. I., Watson, J. S., Heys, S. D., Nevison, I. M., Ritchie, I. K., & Gibson, P. H. (1993). Clenbuterol, aβ-Adrenoceptor Agonist, Increases Relative Muscle Strength in Orthopaedic Patients. Clinical Science, 84(6), 651–654. doi:10.1042/cs0840651
[18] Abdulredha, W. (2019). Effect of Clenbuterol using as weight loose on liver enzymes and lipids profile. Iraq Medical Journal. ISSN 2521-8492.
[19] Kamalakkannan, G., Petrilli, C. M., George, I., LaManca, J., McLaughlin, B. T., Shane, E., … Maybaum, S. (2008). Clenbuterol Increases Lean Muscle Mass but Not Endurance in Patients With Chronic Heart Failure. The Journal of Heart and Lung Transplantation, 27(4), 457–461. doi:10.1016/j.healun.2008.01.013
[20] George I, Xydas S, Mancini DM, et al. Effect of Clenbuterol on cardiac and skeletal muscle function during left ventricular assist device support. J Heart Lung Transplant 2006;25:1084–90.
[21] Yasuda, K., Kawabe, K., Takimoto, Y., Kondo, A., Takaki, R., … Imabayashi, K. (1993). A double-blind clinical trial of a ?2-adrenergic agonist in stress incontinence. International Urogynecology Journal, 4(3), 146–151. doi:10.1007/bf00571623
[22] Lynch, G. S., & Ryall, J. G. (2008). Role of β-Adrenoceptor Signaling in Skeletal Muscle: Implications for Muscle Wasting and Disease. Physiological Reviews, 88(2), 729–767. doi:10.1152/physrev.00028.2007
[23] Durelli, L., Mutani, R., Fassio, F., Satta, A., & Bartoli, E. (1982). Taurine and hyperexcitable human muscle: Effects of taurine on potassium-induced hyperexcitability of dystrophic myotonic and normal muscles. Annals of Neurology, 11(3), 258–265. doi:10.1002/ana.410110305
[24] Jessen, S., Solheim, S. A., Jacobson, G. A., Eibye, K., Bangsbo, J., Nordsborg, N. B., & Hostrup, M. (2019). Beta2‐adrenergic agonist clenbuterol increases energy expenditure and fat oxidation, and induces mTOR phosphorylation in skeletal muscle of young healthy men. Drug Testing and Analysis. doi:10.1002/dta.2755
Author: Type-IIx
Research considerations
Clenbuterol is a drug legally used in human medicine in a select few countries as a bronchodilator at doses up to 40µg daily [18]. Primarily, it is used illicitly as a potent repartitioning agent to promote growth in cattle and sheep by increasing protein accretion and fat removal with little or no change in body weight, and by human athletes for this effect [8].
This work thus makes some extrapolation from animal research on clenbuterol as well as from human research on similar compounds (i.e., salbutamol/albuterol, terbutaline).
Pharmacokinetics
Clenbuterol is a β₂-adrenergic agonist similar in some structural respects to salbutamol (albuterol). Agonism of the β₂ receptor stimulates adenylyl cyclase activity which ultimately leads to downstream effects of smooth muscle relaxation in the bronchioles as a therapeutic target. Clenbuterol is a potent sympathomimetic.
Oral bioavailability of 70-80% and a long half-life of 25-39 hours [6].
Desensitization
Tachyphylaxis, or desensitization, is a feature of the β₂AR. This is likely because β₂AR activation and stimulation of downstream effects are a target for phosphorylation (50) and/or because it binds β-arrestin (51), an accessory protein involved in G protein-coupled receptor desensitization (52) [16].
Tolerability
High doses of clenbuterol have been used in the literature. The highest doses used are for patients with LVAD (left ventricular atrioventricular devices) and congestive heart failure. For this purpose, clenbuterol is used specifically for its effects on cardiac remodeling! It is important to note that cardiac remodeling should generally be avoided by healthy persons (though the remodeling from clen is ostensibly not maladaptive)! There was no change in LV mass with 80 µg daily for 3 months, [19].
Otherwise, an important research consideration is that tolerability cannot be assessed nor inferred from dosages administered in studies. Rather, tolerability must be assessed as an endpoint in the trial, in order to be able to make a claim regarding the drug's tolerability.
Here, this author presents some actual data on tolerability where it has been assessed as an end-point.
From a study on patients with heart failure. Here, the dosage started at 20µg twice daily and was titrated up to 40µg twice daily after a week [19]. Two of the nineteen (2/19) subjects dropped out of the 3 months-long study due to clenbuterol side effects:
Clenbuterol at 80µg/day was well tolerated [19].
Two clenbuterol subjects required discontinuation of study drug (asymptomatic slow ventricular tachycardia, severe muscle cramps without significant elevation increatine kinase [CK]) [19].
One further clenbuterol subject had a high rate of ventricular ectopy that disappeared without reduction in the clenbuterol dose [19].
One placebo subject had frequent non-sustained ventricular tachycardia. There were no implantable cardioverter-defibrillator discharges during the course of the study [19].
Six clenbuterol and 2 placebo subjects reported mild muscle cramps [19].
The CK value was elevated in 5 clenbuterol and 4 placebo subjects. The range of peak CK was 300 to 597 mg/dl in clenbuterol subjects and 305 to 408 mg/dl with placebo. Three clenbuterol subjects had cramps without elevation of CK, and CK was elevated in 1 clenbuterol subject who was asymptomatic. Of importance was that the CK level decreased despite continued drug administration (Figure 1B) [19].
Tremors were reported in 5 clenbuterol and 2 placebo subjects [19].
In a large, double-blind clinical trial (n=175 women with stress incontinence, 82 received 40µg clen daily for 2 weeks):
Side effects were noted in 13 clenbuterol-administered patients (15.9%), and the treatment was discontinued in 5 of these (due to tremors of the finger in 2 cases; dizziness in 1; urinary hesitancy in 1; and loss of appetite in 1). In the remaining 8 patients the side effects were mild and the treatment was able to be completed. In the clenbuterol group, the main side effect was finger tremors (8 out of 82, 9.8%), and tachycardia was also noted (2 out of 82, 2.4%) [20].
In the placebo group, side effects were noted in 12 patients (12.9%), and the treatment was discontinued in 4 of these (dizziness in two patients and nausea in two. One of these 4 patients was treated for longer than 1 week, and the data were thus included in the statistical analysis). In the placebo group, the main side effects were gastrointestinal disturbance (7 out of 93, 7.5%) and dizziness (3 out of 93, 3.2%) [20].
Side effects
- Electrolyte disturbance (primarily hypokalemia and hyperglycemia) and associated muscle cramping
- Tachycardia
- Dyskinesia
- Tremor
- Liver failure
- Muscle atrophy
- Myocardial infarction
- Myocardial reperfusion injury
Cardiac effects
The infamous rat study
"myotoxic" cardiac necrosis
Provides evidence of cardiotoxicity with high doses in rats:
Burrinston et al. (21) explored the myotoxic effects of clenbuterol injected subcutaneously. Rats were treated with a single shot of clenbuterol. The amount of necrosis in the muscle was measured using an anti-myosin monoclonal antibody that enters the endoplasmic reticulum only in cells that have undergone necrosis. The amount of necrosis in the heart was positively correlated with the amount of clenbuterol that was injected. Necrosis was seen at 4 hours post administration and peaked at 15 hours. This finding can explain the cardiomyocyte damage seen after a single ingestion of clenbuterol. Necrosis decreased by 91-100% in rats pre-treated with bisoprolol, β2 blockade or noradrenaline depletion. This finding is consistent with direct myotoxic effect of clenbuterol. Peak cardiomyocyte necrosis was noted 2.4 mm from the apex. This finding is consistent with the high incidence of ECG and echocardiographic changes in the in the inferior and lateral wall in the patients presented. Direct myocardial injury due to catecholamine activation has been well described and attributed to supply/demand imbalance induced by the sustained activation of adrenergic receptors and mitochondrial dysfunction (22) [6].
These pathological changes are not to be taken lightly. Clen causes real cardiac harm at performance- and physique- enhancement dosages.
Interestingly,
[9].[Clenbuterol-induced myocyte necrosis] appear to be driven through clenbuterol-induced pre-synaptic release of catecholamines within the myocardium which cause myocyte damage through a β1-specific mechanism. Such effects can be blocked in vivo by the use of β1-specific antagonists [e.g., bisoprolol].
Case reports of real myocardial injury
See [6].
A common theme of absurdly high doses.
Mechanisms of myocardial injury
cardiac necrosis
[Clenbuterol-induced myocyte necrosis] appear to be driven through clenbuterol-induced pre-synaptic release of catecholamines within the myocardium which cause myocyte damage through a β1-specific mechanism. Such effects can be blocked in vivo by the use of β1-specific antagonists [e.g., bisoprolol] [9].
apoptosis = cell death
The lowest dose of clenbuterol to induce cardiomyocyte apoptosis was 1 microg/kg, with peak apoptosis (0.35 +/- 0.05%; P < 0.05) occurring in response to 5 mg/kg. In the soleus, peak apoptosis (5.8 +/- 2%; P < 0.05) was induced by the lower dose of 10 microg/kg. Cardiomyocyte apoptosis was detected throughout the ventricles, atria, and papillary muscles. However, this damage was most abundant in the left ventricular subendocardium at a point 1.6 mm, that is, approximately one-quarter of the way, from the apex toward the base. beta-AR antagonism (involving propranolol, bisoprolol, or ICI 118551) or reserpine was used to show that clenbuterol-induced myocardial apoptosis was mediated through neuromodulation of the sympathetic system and the cardiomyocyte beta1-AR...
[15]
cardiac hypertrophy
In rat cardiocytes (heart cells), Clenbuterol is associated with a threefold increase in IGF-I mRNA expression. An increase in ANP, BNP, but not αSkA indicates physiological cardiac hypertrophy. Cardiac fibroblasts contain essentially only β2 receptors and are the prime targets of clenbuterol indicating a paracrine signalling role for physiological cardiac hypertrophy. Hypothesis that the β₂-AR physiological function is myocardial protection against stress [9].
? Liver effects
Liver enzymes level may be raised in T+Clen group due to elevation of anabolic process in muscles and raises the activity of liver enzymes to produce amino acids which is needed in this process. Another reason for increase in the liver enzymes may be due to the thermogenic chemicals reaction of Clenbuterol by increasing body metabolism rate, causing organ and body system to function quicker and longer, then liver enzymes work more than normal and this elevation causes overload on liver. Such a result is noticed through raising AST, ALT, and ALP levels in this study. Free radicals or reactive oxygen species (ROS) are accumulated at repetitive muscle contraction (24) or other stress can lead to oxidative stress and related tissue damage. (25, 26) [18].
Clenbuterol effects on liver cell by making lesion on it (27) because of its ability to form ROS (28) which leads to injury of hepatocellular and increasing the level of liver enzymes in blood [18].
Exercise has various effects on liver function enhancing both nutrient metabolism and antioxidant capacity. (29) In addition, exercise is increasing injury of liver cell. (30) This happened because exercise may cause hypoxia by decreasing blood flow in the liver (31) which causes or promotes an adverse effect from free radicals and lipid peroxidation. (32) Exercise-induced muscle injuries involve oxidative burst from immune cells leading to rapid ROS formation and subsequent oxidative damage. (33) For all these reasons, there is elevated liver enzymes in T No CLEN) group when compared with control group [18].
The results from this poor study showed a significant between-group difference (Clen+Training vs. Training) in ALT elevation, with the Clen+Training group showing a higher (significant) elevation in ALT [18]. The (apparent) cross-sectional study ("all groups followed a special diet program... rich in protein, moderate carbohydrate with low fats"... "training in gym with special training program put and designed by their coach [not described]. The "cycle" was absurd (See #Abdulredha protocol). It is not completely clear whether the study is a crossover or an interventional design given that they used statistical methods (repeated latin square design) and made a curious statement "...22 male who are training plus taking Clenbuterol (T+Clen.) (NOTE: take it by their will.)" The authors of this paper fail to consider whether their small group sample for Clen+Training contains an AAS user (which would actually be expected to impact these liver values and lipids. The repeated latin square design carries a risk of the carry-over effect, which certainly applies with training for all variables measured.
Skeletal muscle hypertrophy ↑

cAMP signaling and mechanisms of action downstream of clen's β2AR agonism
[3]
Mechanisms of skeletal muscle hypertrophy
--------------------------------------------
β2-agonists
Epinephrine interacts with the β2 adrenergic receptor (β2AR), a G protein-coupled receptor coded by the ADRB2 gene, which is the most abundant adrenergic receptor present in muscle fibers.
- β2 agonists (e.g., clenbuterol) =[binds]=> β2AR ⇒ activates adenylate cyclase ⇒ ↑cAMP, protein kinase A (PKA)
- Chronic treatment (e.g., clenbuterol) leads to hypertrophy through poorly defined pathways appearing to involve the IGF1-PI3K-Akt-mTOR cascade.
- PKA-dependent phosphorylation of the transcription factor CREB (cAMP response element binding protein) and associated
coactivators play a largely unknown role
- MEF2 (+) [pro-hypertrophic coactivator] may be involved
------------------------------------------

[10]
Attenuation of myostatin has been highlighted as an important factor by which β2-agonists elicit their hypertrophic effects (8, 33, 54, 55). Another factor could be follistatin, which was recently shown to be associated with hypertrophy in rodents following treatment with the β2-agonist formoterol (8). The β2-adrenergic hypertrophic response has also been shown to vary between slow- and fast-twitch muscles of animals (21, 66, 81) [12]. In humans:
A significant interaction (treatment x time) was observed in expression of follistatin between TER and PLA with the intervention. In TER, expression of follistatin was higher (P0.01) after the intervention compared with before, whereas no changes were observed in PLA. Expression of myostatin did notchange with the intervention in either group (Fig. 5B) [12].
Change in single fiber cross-sectional area of myosin heavy chain (MHC) I (1,205 ± 558µm²;P0.01) and MHC II fibers (1,277 ± 595µm²;P0.05) of the vastus lateralis muscle was higher..., whereas no changes were observed in MHC isoform distribution. Expression of muscle proteins involved in growth, ion handling, lactate production, and clearance increased (P0.05) with the intervention (max cycle ergometer) with no change in oxidative enzymes. Our observations suggest that muscle hypertrophy is the primary mechanism underlying enhancements in muscle force and peak power during maximal cycling induced by chronic β2-adrenergic stimulation in humans. [12].
Abo et al. (1) observed that expression of myostatin was higher after 21 days of clenbuterol treatment with no differences in the first week of treatment, suggesting that myostatin functions as a negative regulator in the latter stages of β2-agonist treatment. Nonetheless, while the present observations suggest that follistatin may play a role in β2-adrenergic mediated hypertrophy, the mechanisms are complex and possibly involve an interplay of several factors that directly or indirectly attenuate the negative regulatory action of the myostatin system on growth (8, 54, 55, 72, 75) [12].
mTOR phosphorylation
Human skeletal muscle mTOR phosphorylation ↑121% (potent activator) [24].
Strength, sprint, power ↑
In the overall analyses on healthy non-asthmatic subjects, prohibited β2-agonists (note: clen was not the subject of any study, rather oral salbutamol (albuterol) would be a prohibited agent studied) improved anaerobic performance (0.46 above the expected mean) related to dose (e.g., prohibited) and route of administration (e.g., oral), and a tendency toward greater effect with multiple weeks of treatment [4]. This corresponded to a 70 m sprint time and MVC improvement by 5% in competitive athletes and high performance cyclists and triathletes, respectively. The % improvement was 3% in sprint and 6% in strength in the respective populations [4].
In a study of maximal cycling sprint performance:
Enhancements in muscle force and power output during 30 s of maximal cycling induced by chronic β2-adrenergic stimulation in humans primarily are explained by skeletal muscle hypertrophy. In addition, that change in amount of MHC IIa isoforms and in expression of proteins involved in lactate production (LDH), Ca²⁺ uptake (SERCAI), and oxidative phosphorylation (OXPHOS complex V) of skeletal muscle mediated by chronic β2-adrenergic stimulation were complementary mechanisms for enhancements in power output during 30 s of maximal cycling [12].
We observed that the increase in lean body mass induced by terbutaline treatment was 3% when measured by DXA-scan, whereas the increase observed in CSA of muscle fibers was 13–15% [12].
Peak power and mean power:
Significant interactions (treatment x time) were observed for peak power (P0.01) and mean power (P0.01) between TER and PLA with the intervention. Peak power and mean power increased by 32 ± 8 and 25 ± 9W, respectively. No changes were observed in VO₂max and time to fatigue during incremental cycling with the intervention in either group [12].
Clen, postoperatively, increased leg strength (knee extensor maximum voluntary isometric force) at 40µg daily (20µg 2x) for 6 weeks [17]. While the unoperated leg (consider bilateral strength connection, loss of strength for a long duration prior to study) rebounded in strength in the PLA group (+12N, 1.56%, E.S. 0.44), there was a significant increase in the EXP group of +78N, 10.26%, E.S. 2.29 for clen [17]. There were insufficient participant subjects due to equipment failure for a measure of muscle CSA to draw conclusions from (type 2 error sampling likely; computed tomography insensitive). Strength was maintained throughout the two week washout period [17] (distinguish from salbutamol [albuterol]).
Mechanisms in strength and power increase
There was also a 27% increase in maximal strength that did not reach significance in this small cohort; however, clenbuterol was associated with a decrease in endurance. This paradoxic effect of clenbuterol on strength and endurance was also seen in our prior LVAD study (18) and is consistent with animal experimentation that demonstrated a shift from slow to fast twitch fibers and a transition from oxidative to glycolytic metabolism during clenbuterol treatment. (22,23) [19].
Muscle contractile properties
Maximum voluntary contraction (MVC) and Peak Twitch Force:
Significant interactions (treatment x time) were observed for MVC (P0.01) and peak twitch force (P0.01) between TER and PLA with the intervention. TER increased MVC by 97 ± 29 N and peak twitch force by 67 ± 14 N compared with PLA. Degree of voluntary activation level, time-to-peak twitch force, and half-relaxation time did not change with the intervention in either group [12].
Body composition
↑ Lean body mass and ↓ fat mass:
Significant interactions (treatment x time) were observed for lean body mass (P0.05) and fat mass (P0.05) between TER and PLA with the intervention. TER increased lean body mass by 1.95 ± 0.8 kg and reduced fat mass by 0.97 ± 0.44 kg compared with PLA with the intervention. Body mass and bone mineral density did not change with the intervention in either group. [12].
We observed that the increase in lean body mass induced by terbutaline treatment was 3% when measured by DXA-scan, whereas the increase observed in CSA of muscle fibers was 13–15% [12].
Mechanisms in lipolysis
Upon activation of the β2-adrenoreceptor on adipocytes (fat cells) located in the plasma membrane:
(25-28) activity of the effector units of adenylate cyclase is modulated by a positive effect of the stimulatory guanine nucleotide-binding protein (Gs protein), or a negative effect of the inhibitory guanine nucleotide-binding protein (Gi protein) α subunits of the signal transducing proteins (29, 30) located at the cytoplasmic surface of the plasma membrane. The activation of adenylate cyclase by the Gαs protein generates elevated levels of 3',5'-cyclic adenosine monophosphate (cAMP) from ATP. cAMP activates PKA by dissociating the complex of the regulatory and catalytic subunits (31-34). The caalytic subunit of PKA, in turn, phosphorylates different proteins; one is HSL and another is perilipin at the surface of lipid storage droplets (37,38). On phosphorylation, HSL translocates from the cytosol to the lipid droplet surface, which becomes accessible to hydrolysis because PKA phosphorylation alleviates the barrier function of perilipin (39-42). Finally, triglycerides are hydrolyzed to glycerol and FFAs [13].
RMR increase
RMR increase in humans:
80 μg clenbuterol ↑RMR 21% over 3 hr (78 kg bodyweight men), fat oxidation ↑39% [24].
Metabolism
↑plasma concentrations of glucose (+25%), lactate (+87%), insulin (+105%), fatty acids (+129%) [24].
Oxidative capacity
No diminished oxidative capacity in humans.
In the present study, however,we observed no effect of terbutaline on V ̇O2maxand time tofatigue during incremental exercise, which is consistent withthat observed in endurance athletes after 2-wk treatment of oralsalbutamol (30). Collomp et al. (16) even observed that 3-wktreatment of oral salbutamol increased time to fatigue duringsubmaximal exercise in trained men. Our muscle protein dataalso showed no indication of depressed oxidative capacity,since no changes were observed in CS, HAD, COX4, andOXPHOS complexes III-V with terbutaline. In fact, we ob-served that terbutaline increased muscle expression of PDH aswell as LDH and MCT1, which is essential for lactate produc-tion and clearance during exercise. Likewise, muscle expres-sion of Na-K-ATPase subunits2and1and FXYD1 washigher after terbutaline treatment in the present study. [12].
Endogenous GH effects
β-adrenergic blockade/antagonism enhances the GH response to GHRH (secreted by the hypothalamus) but has no apparent effect on spontaneous GH secretion (Muller, 1987; Guistina & Veldhuis, 1998; Martha, Blizzard & Rogol, 1988) [11].
Administration of salbutamol (albuterol) [i.e., clenbuterol], a β₂-adrenergic agonist, inhibits GH secretion and is able to block the stimulation of GH release by L-arginine or pyridostigmine (Ghigo et al., 1994) [11].
Other pharmacological agents, receptors, and putative mechanisms
Nicotinic cholinergic and α₁-adrenergic receptors appear to have lesser effects on GH secretion (Muller, 1987; Guistina & Veldhuis, 1998) [11]. Antagonists of α₂-adrenergic receptors (e.g., yohimbine) can completely block the stimulatory effects on GH secretion of enhancing cholinergic tone with pyridostigmine, a cholinesterase inhibitor (Devesa et al., 1991) [11]. Administration of the α₂-adrenergic agonist clonidine stimulates GH secretion (Miki, Ono, & Shizume, 1984) [11].
Conclusion: Most experimental evidence supports the hypothesis that activation of β-adrenergic receptors increases hypothalamic somatostatin secretion (Guistina & Veldhuis, 1998) [11].
Adjunct drugs
rhGH ✓
Since the primary purpose of clen is lipolysis/recomp it makes a great deal of sense to use rhGH for its additional and potent effects for the same goal.
rhGH exerts its lipolytic effect via multiple pathways including some regulation of the adrenergic system. Some evidence suggests increased beta1 and beta3 receptor function [13] - indicating potential synergism (albeit without a reduction in the effective dose of clen) due to effects on different mediators of lipolysis.
Further, clen as a β-adrenergic agonist inhibits endogenous GH secretion [11] indicating strongly for the administration of exogenous (rh)GH.
++ additional lipolytic/recomp effects
++ ameliorates the inhibition of endogenous GH secretion
Taurine ✓
Muscle cramps due to electrolyte imbalance (hypokalemia and hyperglycemia mostly) may be ameliorated by oral administration of Taurine.
The diet supplements containing taurine function by replacing the missing nutrients in the body. Taurine, as a single agent, presents different functions like substrate for formation of bile salts, cell volume regulation, modulation of intracellular calcium, cytoprotection of central nervous system, etc. (PubChem)
Oral administration of taurine in healthy individuals gave a plasma elimination half-life that ranged from 0.7-1.4 h [14]. In healthy individuals a clearance rate that ranged from 14 to 34.4 L/h [14].
+ modulation of intra- and trans- cellular electrolytes in skeletal muscle
Putative mechanism
Repetitive activity would be sustained by potassium accumulation in the T-tubular system (1, 13, 14), which would in turn keep the membrane potential sufficiently depolarized for potassium conductance to be inactivated through the anomalous rectification mechanism (6). The rhythmic inactivation of potassium conductance would probably account for repetitive action potentials (6). The depressant action of taurine upon excitable cells has been largely substantiated in studies of neural, retinal, or cardiac tissues (11, 37, 39), but only scattered experiments have focused on the effects of this amino acid upon skeletal muscles (5, 18). Taurine has been shown to hyperpolarize muscle or nerve cells (18,23), and this effect can be ascribed to an increment of intracellular potassium concentration (18, 40) as well as to an increase of potassium and chloride conductances, possibly by modulation of the availability of intracellular calcium (11, 25). [23]
Bisoprolol, Metoprolol ✓
β1 specific antagonist
Bisoprolol is an antihypertensive drug routinely prescribed in medicine with specific antagonism of the β1-AR and reduction in the RAAS.
The mechanism by which clen may induce cardiac necrosis involves catecholamine activation of the β1-adrenergic receptors [9]. In order to prevent this myotoxic effect, a β1 antagonist, bisoprolol, should be used if accessible.
Though the mechanism of action of bisoprolol has not been fully elucidated in hypertension, it is thought that therapeutic effects are achieved through the antagonism of β-1adrenoceptors to result in lower cardiac output. Bisoprolol is a competitive, cardioselective β1-adrenergic antagonist. When β1-receptors (located mainly in the heart) are activated by adrenergic neurotransmitters such as epinephrine, both the blood pressure and heart rate increase, leading to greater cardiovascular work, increasing the demand for oxygen. Bisoprolol reduces cardiac workload by decreasing contractility and the need for oxygen through competitive inhibition of β1-adrenergic receptors. Bisoprolol is also thought to reduce the output of renin in the kidneys, which normally increases blood pressure. Additionally, some central nervous system effects of bisoprolol may include diminishing sympathetic nervous system output from the brain, decreasing blood pressure and heart rate. (DrugBank)
++ prevents cardiac toxicity
The evidence does not support the use of any of these following drugs in combination with clen to counter the β-adrenergic receptor downregulation:
Ketotifen ✖
Ketotifen is a relatively selective, non-competitive histamine antagonist (H1-receptor) and mast cell stabilizer. Ketotifen inhibits the release of mediators from mast cells involved in hypersensitivity reactions. Decreased chemotaxis and activation of eosinophils have also been demonstrated. Ketotifen also inhibits cAMP phosphodiesterase. Properties of ketotifen which may contribute to its antiallergic activity and its ability to affect the underlying pathology of asthma include inhibition of the development of airway hyper-reactivity associated with activation of platelets by PAF (Platelet Activating Factor), inhibition of PAF-induced accumulation of eosinophils and platelets in the airways, suppression of the priming of eosinophils by human recombinant cytokines and antagonism of bronchoconstriction due to leukotrienes. Ketotifen inhibits of the release of allergic mediators such as histamine, leukotrienes C4 and D4(SRS-A) and PAF.
Ketotifen is a non-bronchodilator antiasthmatic drug which inhibits the effects of certain endogenous substances known to be inflammatory mediators, and thereby exerts antiallergic activity. Ketotifen possesses a powerful and sustained non-competitive histamine (H1) blocking property. Ketotifen's antihistamine (H1) effect seems to be distinct from it antiallergic properties. Properties of ketotifen which may contribute to its antiallergic activity and its ability to affect the underlying pathology of asthma include: In Vivo results: Inhibition of the development of airway hyperreactivity associated with activation of platelets by PAF (Platelet Activating Factor) or caused by neural activation following the use of sympathomimetic drugs or the exposure to allergen; inhibition of PAF-induced accumulation of eosinophils and platelets in the airways; suppression of the priming of eosinophils by human recombinant cytokines and thereby suppression of the influx of eosinophils into inflammatory loci; antagonism of bronchoconstriction due to leukotrienes. In Vitro results: inhibition of the release of allergic mediators such as histamine, leukotrienes C4 and D4 (SRS-A) and PAF. /Ketotifen fumarate (systemic)/ (PubChem)
- Inhibits cAMP activity (clen's primary target of action)
Yohimbine ✖
Yohimbine is a pre-synaptic alpha 2-adrenergic blocking agent. The exact mechanism for its use in impotence has not been fully elucidated. However, yohimbine may exert its beneficial effect on erectile ability through blockade of central alpha 2-adrenergic receptors producing an increase in sympathetic drive secondary to an increase in norepinephrine release and in firing rate of cells in the brain noradrenergic nuclei. Yohimbine-mediated norepinephrine release at the level of the corporeal tissues may also be involved. In addition, beneficial effects may involve other neurotransmitters such as dopamine and serotonin and cholinergic receptors. (PubChem)
- Inhibits GH secretion
- further increases sympathetic drive
- The risk or severity of hypertension can be increased when Yohimbine is combined with Clenbuterol (DrugBank)
+ synergistic/additive lipolytic effect possible via action at different (α₂-adrenergic) receptors
Benadryl ✖
Diphenhydramine
- The risk or severity of Tachycardia can be increased when Diphenhydramine is combined with Clenbuterol. (DrugBank)
Practical
Llewellyn protocol
...Copyright...
Abdulredha protocol
This protocol comes from an actual (terrible) research study [18] from the Iraq Medical Journal (2019). Its namesake is its progenitor, Dr. Abdulredha. Do not use this protocol: it is an example of atrociously bad research.
Dr. Abdulredha proposes 6 cycles (12 weeks) of the following protocol:
Day 1: 20µg, Day 2: 40µg, Day 3: 60µg, Day 4: 80µg, Day 5: 100µg, Day 6: 120µg, Day 7: 140µg
Day 8: 140µg, Day 9: 120µg, Day 10: 100µg, Day 11: 80µg, Day 12: 60µg, Day 13: 40µg, Day 14: 20µg
[18]
Modern protocol
I have a great deal to say about my extrapolation (and implementation) of practical use from the data on clen, beta2-adrenergic agonists, and PEDs generally.
I am now offering consulting for a fee for individualized performance- and physique- enhancement protocols and answering all questions, with support for all statements made with references and explanation of logic, on any and all questions related to the science and practice of PEDs, with live chat scheduled.
If you are serious about consulting, you may contact me here via PM with the message titled “Consulting” and we can discuss.
______________________________
References:
[1] GEORGE, I., XYDAS, S., MANCINI, D., LAMANCA, J., DITULLIO, M., MARBOE, C., … PETRILLI, C. (2006). Effect of Clenbuterol on Cardiac and Skeletal Muscle Function During Left Ventricular Assist Device Support. The Journal of Heart and Lung Transplantation, 25(9), 1084–1090. doi:10.1016/j.healun.2006.06.017
[2] YAMAMOTO, I., IWATA, K., & NAKASHIMA, M. (1985). Pharmacokinetics of plasma and urine clenbuterol in man, rat, and rabbit. Journal of Pharmacobio-Dynamics, 8(5), 385–391. doi:10.1248/bpb1978.8.385
[3] Parr MK, Müller-Schöll A. Pharmacology of doping agents—mechanisms promoting muscle hypertrophy. AIMS Molecular Science 2018;5:145-55.
[4] Riiser A, Stensrud T, Stang J, Andersen LB. Can β2-agonists have an ergogenic effect on strength, sprint or power performance? Systematic review and meta-analysis of RCTs. Br J Sports Med. 2020 Nov;54(22):1351-1359. doi: 10.1136/bjsports-2019-100708. Epub 2020 Aug 3. PMID: 32747344.
[5] Schiaffino, S., Reggiani, C., Akimoto, T., & Blaauw, B. (2020). Molecular Mechanisms of Skeletal Muscle Hypertrophy. Journal of Neuromuscular Diseases, 1–15. doi:10.3233/jnd-200568
[6] Shafrir A, Leibowitz DW, Alcalai R, Elitzur Y, Muszkat M. Myocardial injury induced by the long acting beta2 adrenergic agonist clenbuterol. Cardiol Cardiovasc Med 2019;3(4):186–192.
[7] Witkowska-Piłaszewicz O, Pingwara R, Szczepaniak J, Winnicka A. The Effect of the Clenbuterol-β2-Adrenergic Receptor Agonist on the Peripheral Blood Mononuclear Cells Proliferation, Phenotype, Functions, and Reactive Oxygen Species Production in Race Horses In Vitro. Cells. 2021 Apr 17;10(4):936. doi: 10.3390/cells10040936. PMID: 33920705; PMCID: PMC8072563.
[8] SLEEPER, M. M., KEARNS, C. F., & McKEEVER, K. H. (2002). Chronic clenbuterol administration negatively alters cardiac function. Medicine & Science in Sports & Exercise, 34(4), 643–650. doi:10.1097/00005768-200204000-00013
[9] Bhavsar, P. K., Brand, N. J., Felkin, L. E., Luther, P. K., Cullen, M. E., Yacoub, M. H., & Barton, P. J. R. (2010). Clenbuterol Induces Cardiac Myocyte Hypertrophy via Paracrine Signalling and Fibroblast-derived IGF-1. Journal of Cardiovascular Translational Research, 3(6), 688–695. doi:10.1007/s12265-010-9199-1
[10] Meyer, H. H. D. (2001). Biochemistry and physiology of anabolic hormones used for improvement of meat production. APMIS, 109(1), 1–8. doi:10.1111/j.1600-0463.2001.tb00010.x
[11] Growth Hormone in Adults: Physiological and Clinical Aspects, Second Edition. 2000.
[12] Hostrup, M., Kalsen, A., Onslev, J., Jessen, S., Haase, C., Habib, S., … Bangsbo, J. (2015). Mechanisms underlying enhancements in muscle force and power output during maximal cycle ergometer exercise induced by chronic β2-adrenergic stimulation in men. Journal of Applied Physiology, 119(5), 475–486. doi:10.1152/japplphysiol.00319.2015
[13] Yang S, Mulder H, Holm C, Edén S. Effects of growth hormone on the function of beta-adrenoceptor subtypes in rat adipocytes. Obes Res. 2004 Feb;12(2):330-9. doi: 10.1038/oby.2004.41. PMID: 14981226.
[14] Ghandforoush-Sattari M, Mashayekhi S, Krishna CV, Thompson JP, Routledge PA: Pharmacokinetics of oral taurine in healthy volunteers. J Amino Acids. 2010;2010:346237. doi: 10.4061/2010/346237. Epub 2010 Jun 29.
[15] Burniston JG, Tan LB, Goldspink DF. beta2-Adrenergic receptor stimulation in vivo induces apoptosis in the rat heart and soleus muscle. J Appl Physiol (1985). 2005 Apr;98(4):1379-86. doi: 10.1152/japplphysiol.00642.2004. Epub 2004 Dec 10. PMID: 15591297.
[16] Collins, S., Cao, W., Daniel, K. W., Dixon, T. M., Medvedev, A. V., Onuma, H., & Surwit, R. (2001). Adrenoceptors, Uncoupling Proteins, and Energy Expenditure. Experimental Biology and Medicine, 226(11), 982–990. doi:10.1177/153537020122601104
[17] Maltin, C. A., Delday, M. I., Watson, J. S., Heys, S. D., Nevison, I. M., Ritchie, I. K., & Gibson, P. H. (1993). Clenbuterol, aβ-Adrenoceptor Agonist, Increases Relative Muscle Strength in Orthopaedic Patients. Clinical Science, 84(6), 651–654. doi:10.1042/cs0840651
[18] Abdulredha, W. (2019). Effect of Clenbuterol using as weight loose on liver enzymes and lipids profile. Iraq Medical Journal. ISSN 2521-8492.
[19] Kamalakkannan, G., Petrilli, C. M., George, I., LaManca, J., McLaughlin, B. T., Shane, E., … Maybaum, S. (2008). Clenbuterol Increases Lean Muscle Mass but Not Endurance in Patients With Chronic Heart Failure. The Journal of Heart and Lung Transplantation, 27(4), 457–461. doi:10.1016/j.healun.2008.01.013
[20] George I, Xydas S, Mancini DM, et al. Effect of Clenbuterol on cardiac and skeletal muscle function during left ventricular assist device support. J Heart Lung Transplant 2006;25:1084–90.
[21] Yasuda, K., Kawabe, K., Takimoto, Y., Kondo, A., Takaki, R., … Imabayashi, K. (1993). A double-blind clinical trial of a ?2-adrenergic agonist in stress incontinence. International Urogynecology Journal, 4(3), 146–151. doi:10.1007/bf00571623
[22] Lynch, G. S., & Ryall, J. G. (2008). Role of β-Adrenoceptor Signaling in Skeletal Muscle: Implications for Muscle Wasting and Disease. Physiological Reviews, 88(2), 729–767. doi:10.1152/physrev.00028.2007
[23] Durelli, L., Mutani, R., Fassio, F., Satta, A., & Bartoli, E. (1982). Taurine and hyperexcitable human muscle: Effects of taurine on potassium-induced hyperexcitability of dystrophic myotonic and normal muscles. Annals of Neurology, 11(3), 258–265. doi:10.1002/ana.410110305
[24] Jessen, S., Solheim, S. A., Jacobson, G. A., Eibye, K., Bangsbo, J., Nordsborg, N. B., & Hostrup, M. (2019). Beta2‐adrenergic agonist clenbuterol increases energy expenditure and fat oxidation, and induces mTOR phosphorylation in skeletal muscle of young healthy men. Drug Testing and Analysis. doi:10.1002/dta.2755
Last edited:

