Aghazadeh Y, Martinez-Arguelles DB, Fan J, Culty M, Papadopoulos V. Induction of Androgen Formation in the Male by a TAT-VDAC1 Fusion Peptide Blocking 14-3-3varepsilon Protein Adaptor and Mitochondrial VDAC1 Interactions. Mol Ther 2014;22(10):1779-91. http://www.nature.com/mt/journal/v22/n10/full/mt2014116a.html
Low testosterone (T), a major cause of male hypogonadism and infertility, is linked to mood changes, fatigue, osteoporosis, reduced bone-mass index, and aging.
The treatment of choice, T replacement therapy, has been linked with increased risk for prostate cancer and luteinizing hormone (LH) suppression, and shown to lead to infertility, cardiovascular diseases, and obesity.
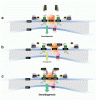
Alternate methods to induce T with lower side effects are desirable. In search of the mechanisms regulating T synthesis in the testes, we identified the 14-3-3varepsilon protein adaptor as a negative regulator of steroidogenesis.
Steroidogenesis begins in mitochondria. 14-3-3varepsilon interacts with the outer mitochondrial membrane voltage-dependent anion channel (VDAC1) protein, forming a scaffold that limits the availability of cholesterol for steroidogenesis.
We report the development of a tool able to induce endogenous T formation. Peptides able to penetrate testes conjugated to 14-3-3varepsilon site of interaction with VDAC1 blocked 14-3-3varepsilon-VDAC1 interactions while at the same time increased VDAC1-translocator protein (18 kDa) interactions that induced steroid formation in rat testes, leading to increased serum T levels.
These peptides rescued intratesticular and serum T formation in adult male rats treated with gonadotropin-releasing hormone antagonist, which dampened LH and T production.
Low testosterone (T), a major cause of male hypogonadism and infertility, is linked to mood changes, fatigue, osteoporosis, reduced bone-mass index, and aging.
The treatment of choice, T replacement therapy, has been linked with increased risk for prostate cancer and luteinizing hormone (LH) suppression, and shown to lead to infertility, cardiovascular diseases, and obesity.
Alternate methods to induce T with lower side effects are desirable. In search of the mechanisms regulating T synthesis in the testes, we identified the 14-3-3varepsilon protein adaptor as a negative regulator of steroidogenesis.
Steroidogenesis begins in mitochondria. 14-3-3varepsilon interacts with the outer mitochondrial membrane voltage-dependent anion channel (VDAC1) protein, forming a scaffold that limits the availability of cholesterol for steroidogenesis.
We report the development of a tool able to induce endogenous T formation. Peptides able to penetrate testes conjugated to 14-3-3varepsilon site of interaction with VDAC1 blocked 14-3-3varepsilon-VDAC1 interactions while at the same time increased VDAC1-translocator protein (18 kDa) interactions that induced steroid formation in rat testes, leading to increased serum T levels.
These peptides rescued intratesticular and serum T formation in adult male rats treated with gonadotropin-releasing hormone antagonist, which dampened LH and T production.