[Essentially, potential targets for testosterone synthesis.]
This study systematically evaluates the effects of androgen receptor (AR) blockade on molecular events in Leydig cells.
Results showed that intramuscular administration of Testosterone-enanthate, at clinically relevant dose, decreased testosterone in interstitial fluid and Leydig cells from adult rats.
AR-blocker (Androcur) prevented this effect and testosterone-reduced Leydig cells steroidogenic capacity/activity.
Testosterone-reduced expression of some steroidogenic enzymes/proteins (Tspo,StAR,Hsd3b1/2) and transcription factors (Nur77,Gata4,Dax1) was completely abrogated, while decreased expression of Star,Cyp11a1,Cyp17a1,Hsd17b4,Creb1a was partially prevented.
In the same cells, increased expression of Hsd3b5/HSD3B and Ar/AR was abolished.
Androcur-treatment abolished testosterone-reduced cAMP, coupled with a changed expressional milieu of cAMP signaling elements.
Results from in vitro experiments suggest that some of these effects are testosterone-AR dependent, while others could be due to disturbed LH and/or other signals.
Presented data provide new molecular insight into Leydig cells function and are important in terms of human reproductive health and the wide-spread use of Androcur as well as use/abuse of Testosterone-enanthate.
Bjelic MM, Stojkov NJ, Baburski AZ, et al. Molecular adaptations of testosterone-producing leydig cells during systemic in vivo blockade of the androgen receptor. Molecular and Cellular Endocrinology. https://www.sciencedirect.com/science/article/pii/S030372071400241X
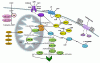
Main signaling pathways and molecules involved in the regulation of Leydig cell function/homeostasis. Steroid hormones in Leydig cells are synthesized from a common precursor, cholesterol.
Transport of cholesterol from intracellular sources into the mitochondria requires the presence of a specific complex of transport carrier proteins (orange): TSPO (translocator protein) and StAR (steroidogenic acute regulatory) protein.
Conversion of cholesterol to testosterone is catalyzed by steroidogenic enzymes (light green): CYP11A1 (cholesterol side-chain cleavage enzyme), HSD3B (3beta-hydroxysteroid dehydrogenase), CYP17A1 (17alpha-hydroxylase/17,20lyase), HSD17B (17beta- hydroxysteroid dehydrogenase).
After biosynthesis, testosterone could be converted to DHT (dihydrotestosterone) or estradiol by specific enzymes (light green): 5alphaRED (5alpha-reductase), CYP19A1 (aromatase).
Testosterone or DHT exert their physiological function through the androgen receptor – AR (dark green).
The steroidogenic function of Leydig cells is predominantly regulated by pituitary luteinizing hormone (LH) or its placental counterpart, human chorionic gonadotropin (hCG), through LHCG receptors (dark blue).
Activation of these receptors leads to stimulation of the cAMP PRKA signaling pathway (yellow): ADCY (adenylyl cyclase), PRKA (cAMP-dependent kinase). Termination of cAMP signaling is mediated mainly by PDE4 (phosphodiesterase 4).
Regulation of Leydig cell steroidogenesis and homeostasis is a multi-compartmental process, which could be regulated governed by complex endocrine, paracrine, and autocrine signals.
Some of these include:
· cGMP signaling pathway (light blue): NOS (nitric oxide synthase), GUCY1 (guanylyl cyclase type 1), PRKG1 (protein kinase G type 1);
· ERK signaling (dark purple): INS/IGF receptors, ERK1/2 (Extracellular signal regulated kinase .);
· Adrenergic receptors signaling (light purple): ADRA/B (alpha and beta adrenergic receptors), PLC (phospholipase C), PKC (protein kinase C), cAMP-PRKA etc.;
· Components of the intrinsic mitochondrial apoptotic pathway (light brown): CYTC (cytochrome C), APAF-1 (apoptotic protease activating factor 1), CASP9 (caspase 9), CASP3 (caspase 3).
This study systematically evaluates the effects of androgen receptor (AR) blockade on molecular events in Leydig cells.
Results showed that intramuscular administration of Testosterone-enanthate, at clinically relevant dose, decreased testosterone in interstitial fluid and Leydig cells from adult rats.
AR-blocker (Androcur) prevented this effect and testosterone-reduced Leydig cells steroidogenic capacity/activity.
Testosterone-reduced expression of some steroidogenic enzymes/proteins (Tspo,StAR,Hsd3b1/2) and transcription factors (Nur77,Gata4,Dax1) was completely abrogated, while decreased expression of Star,Cyp11a1,Cyp17a1,Hsd17b4,Creb1a was partially prevented.
In the same cells, increased expression of Hsd3b5/HSD3B and Ar/AR was abolished.
Androcur-treatment abolished testosterone-reduced cAMP, coupled with a changed expressional milieu of cAMP signaling elements.
Results from in vitro experiments suggest that some of these effects are testosterone-AR dependent, while others could be due to disturbed LH and/or other signals.
Presented data provide new molecular insight into Leydig cells function and are important in terms of human reproductive health and the wide-spread use of Androcur as well as use/abuse of Testosterone-enanthate.
Bjelic MM, Stojkov NJ, Baburski AZ, et al. Molecular adaptations of testosterone-producing leydig cells during systemic in vivo blockade of the androgen receptor. Molecular and Cellular Endocrinology. https://www.sciencedirect.com/science/article/pii/S030372071400241X
Main signaling pathways and molecules involved in the regulation of Leydig cell function/homeostasis. Steroid hormones in Leydig cells are synthesized from a common precursor, cholesterol.
Transport of cholesterol from intracellular sources into the mitochondria requires the presence of a specific complex of transport carrier proteins (orange): TSPO (translocator protein) and StAR (steroidogenic acute regulatory) protein.
Conversion of cholesterol to testosterone is catalyzed by steroidogenic enzymes (light green): CYP11A1 (cholesterol side-chain cleavage enzyme), HSD3B (3beta-hydroxysteroid dehydrogenase), CYP17A1 (17alpha-hydroxylase/17,20lyase), HSD17B (17beta- hydroxysteroid dehydrogenase).
After biosynthesis, testosterone could be converted to DHT (dihydrotestosterone) or estradiol by specific enzymes (light green): 5alphaRED (5alpha-reductase), CYP19A1 (aromatase).
Testosterone or DHT exert their physiological function through the androgen receptor – AR (dark green).
The steroidogenic function of Leydig cells is predominantly regulated by pituitary luteinizing hormone (LH) or its placental counterpart, human chorionic gonadotropin (hCG), through LHCG receptors (dark blue).
Activation of these receptors leads to stimulation of the cAMP PRKA signaling pathway (yellow): ADCY (adenylyl cyclase), PRKA (cAMP-dependent kinase). Termination of cAMP signaling is mediated mainly by PDE4 (phosphodiesterase 4).
Regulation of Leydig cell steroidogenesis and homeostasis is a multi-compartmental process, which could be regulated governed by complex endocrine, paracrine, and autocrine signals.
Some of these include:
· cGMP signaling pathway (light blue): NOS (nitric oxide synthase), GUCY1 (guanylyl cyclase type 1), PRKG1 (protein kinase G type 1);
· ERK signaling (dark purple): INS/IGF receptors, ERK1/2 (Extracellular signal regulated kinase .);
· Adrenergic receptors signaling (light purple): ADRA/B (alpha and beta adrenergic receptors), PLC (phospholipase C), PKC (protein kinase C), cAMP-PRKA etc.;
· Components of the intrinsic mitochondrial apoptotic pathway (light brown): CYTC (cytochrome C), APAF-1 (apoptotic protease activating factor 1), CASP9 (caspase 9), CASP3 (caspase 3).

Last edited:

