Justaguy99
Member
Definitely interested in sales but still no idea if they are holding our Canadian packs, switched couriers etc.
Ill wait for the other 3 first I guess.
Ill wait for the other 3 first I guess.
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
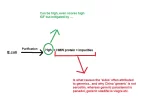
Our HGH is more of an 97%.no they are not not in the field of 'developing' generics.
i dont want to go into science too much but that 5% missing from generics is what gives the side-effects, if something is 95% pharmacetiucal means 5% has deteroitated etc, whereas in generics it means a world lot of difference.
Do you have anything produced at the moment thats 99% and over (or is the cost to 'purify' to expensive for you guys to warrant that)?
Let me know. Regards
All at once.@Qingdao Sigma Chemicals will oil pre orders from Black Friday promo be shipped all at once if different compounds were ordered or send whatever becomes available first.
My promo package is on hold until after Canada post resumes shipping just FYIDefinitely interested in sales but still no idea if they are holding our Canadian packs, switched couriers etc.
Ill wait for the other 3 first I guess.
I was told last week by Bella that there was no Mast E kits left.We have some kits left now.
Puck consistence is quite important, there are specific pharmacopeia specification on how the puck should be. Even so it tests at 99% purity doesn't mean it's all good.This batch is ugly pucks too, you can check the picture in the report, and their ugliness is what explains the 99.9% each time it's tested
Bro im going to try and educate you on the topic because me and the person your talking of have spoken about this and it has come to this (and im not sure if your the manufactorer or middle-man, as as you know purity cost more money to attain).Our HGH is more of an 97%.
You say "if something is 95% pharmacetiucal means 5% has deteroitated etc, whereas in generics it means a world lot of difference."
Which is incorrect, janoshik said many times that the 3-5% you find as impurity in generic HGH is just degraded HGH.
Another proof is that our HGH already tested 99%, so in beginning it's a 99% raw powder, that degrades and stabilize at 97%, the 99% versions are often unstable.
Good luck finding a 99% purity HGH, because simply you won't.
And also to explain that sides comes from impurity, I am sure if you get a 90% purity norditropin and you paid for it a fortune, you will be feeling less sides than a 97% $40 chinese generic kit, because it's all in the head.
NiceTout à coup.
Mais si un acheteur commande par exemple uniquement les premiers prêts, il obtient son suivi avant les autres.
Par exemple, pour les gars qui ont commandé les kits de test électroniques uniquement pendant les précommandes, leurs suivis sont déjà disponibles pour la plupart.
Mais c'est rapide, cette semaine je crois qu'ils seront tous prêts.
Les nouveaux lots sont conditionnés en flacons de 15 ml remplis par machine avec une cible de 11 ml environ.
Verre plus épais.
Nouveaux bouchons en caoutchouc.
Pas de flotteurs.
Le lot Primo 100 gris était l'un de ces nouveaux lots.
Nous comptons sur vous pour faire des retours sur les nouveaux lots et s'ils sont améliorés.
Les échantillons seront également envoyés pour des tests.
Send an email to tracy@sigma email for thisStill no tracking on raws promo payment made 2 weeks ago. Are these actually shipping out domestic? Was this stuff actually on the ground ready to ship? @Qingdao Sigma Chemicals
RecombinantHello qsc
Is your HCG recombinant or from bacteria etc or from human sources like Ovitrelle in France wich is frome urine ? Thanks
I haven’t update the price list and it’s gonna take me some time to do itI was told last week by Bella that there was no Mast E kits left.
if we proceeded with the purchase and payment of the test promo and then bought other oils during the blackfriday promo but in the continuation of this email, we receive a package from the test promo then after a package with the blackfriday promo is that it?Send an email to tracy@sigma email for this
Can confirm the new vials and caps/stoppers are much improved.All at once.
But if a buyer ordered for example the first ready ones only, he gets his tracking before the rest.
For example the guys who ordered the test e kits only during the preorders their trackings are already available for most.
But it’s quick, this week I believe all of them will be ready.
New batchs are in 15ml vials filled by machine with a 11ml target or so.
Thicker glass.
New rubber stoppers.
No floaters.
Primo 100 grey batch was one of these new batchs.
We count on you to make the feedbacks on the new batchs and if they are improved.
The samples will be sent also for testing.
Bro im going to try and educate you on the topic because me and the person your talking of have spoken about this and it has come to this (and im not sure if your the manufactorer or middle-man, as as you know purity cost more money to attain).
View attachment 305746

