James have you tried other peptides, for eg CJC or GHRP 6, and did you see any difference ?
Yes, I have tried Ipamorelin and Mod GRF 1-29, but those have not made in difference in libido.
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
James have you tried other peptides, for eg CJC or GHRP 6, and did you see any difference ?
The last appointment I mentioned took the 'wait another 3 months and see' approach...
I'm now 7 months off treatment and feeling the same.... Have an appointment in two weeks, hopefully it wont be a repeat of the above... I am hoping for a gel trial or monotherapy with an AI (NHS)
A PATIENT WITH ELEVATED TESTOSTERONE AND GONADOTROPINS: IS IT SECONDARY HYPERGONADISM? [Abstract #1004] http://am.aace.com/sites/all/files/Abstract-Book.pdf
..
Conclusion: Patients with chronic liver disease may have elevated SHBG causing high total testosterone and estradiol. Free estradiol may become so low that it loses its ability to suppress gonadotropins. The resulting elevated gonadotropins and high total testosterone may mimic a picture of secondary hypergonadism. A partial testosterone resistance can’t be ruled out as well.
Highlights
· Hyperinsulinemia does not downregulate hepatic SHBG production in obesity.
· Proinflammatory cytokines and hepatic steatosis downregulate SHBG in obesity.
· Monosaccharides and palmitate downregulate and oleate upregulates hepatic SHBG.
· Plasma SHBG could be a biomarker of the degree of inflammation in metabolic disorders.
Simo R, Saez-Lopez C, Barbosa-Desongles A, Hernandez C, Selva DM. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab. https://www.sciencedirect.com/science/article/pii/S1043276015000831
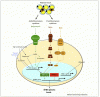
Sex hormone-binding globulin (SHBG) is produced and secreted by the liver into the bloodstream where it binds sex steroids and regulates their bioavailability.
Traditionally, body mass index (BMI) was thought to be the major determinant of SHBG concentrations and hyperinsulinemia the main cause for low SHBG levels found in obesity.
However, no mechanisms have ever been described. Emerging evidence now shows that liver fat content rather than BMI is a strong determinant of circulating SHBG.
In this review we discuss evidence demonstrating that insulin might not regulate SHBG production, describe putative molecular mechanisms by which proinflammatory cytokines downregulate SHBG, and comment on recent findings suggesting dietary SHBG regulation. Finally, clinical implications of all of these findings and future perspectives are discussed.